On Oct. 29, the U.S. Food and Drug Administration (FDA) granted emergency use authorization for the Pfizer-BioNTech (Pfizer) COVID-19 vaccine for those 5 to 11 years of age.[1]Health Canada followed with its own approval on Nov. 19.[2]
These approvals were based on analysis of the vaccine’s effectiveness and safety. The number of participants in the trial for children was much lower than in the trial for adults, and a technique called immunobridging was used to provide additional confidence in the drug’s effectiveness.[3]
This article describes key elements of the Pfizer trial.
The Pfizer vaccine trial for children – what is different?
The format of the children’s trial was similar to previous studies, including the study of individuals aged 16 and over, which was approved by the FDA on Dec. 11, 2020, and the amendment for individuals aged 12 years and older that was approved on May 10, 2021. The trial follows a two-dose series.[4]
The key differences in the children’s trial:
- Dosage – instead of the 30 microgram (mg) doses used for adults, children will receive 10-mg doses. This was determined from safety and effectiveness analysis in Phase 1 of the trial.
- Numbers in the trial – the children’s Phase 2/3 trial had 2,268 participants, while the original adults trial used 43,448 participants.[5]
- Immunobridging. To make up for the small number of test participants, the trial used supplementary immunobridging techniques to infer vaccine effectiveness.
Estimated vaccine effectiveness for children
We discussed vaccine effectiveness (VE) in the article Understanding vaccine effectiveness and breakthrough . The arrangement of the VE formula used for the vaccine trial is shown below, and is calculated using the relative difference in incidence rate of being vaccinated versus unvaccinated:
Media reports of the Phase 2/3 trial results vary by about 1% based on the number of participants deemed eligible in the trial, and the defined endpoints in time. Our estimate uses all participants who became infected and yields a 91.3% vaccine effectiveness. This effectiveness is similar to Pfizer’s tests on adults.[6]
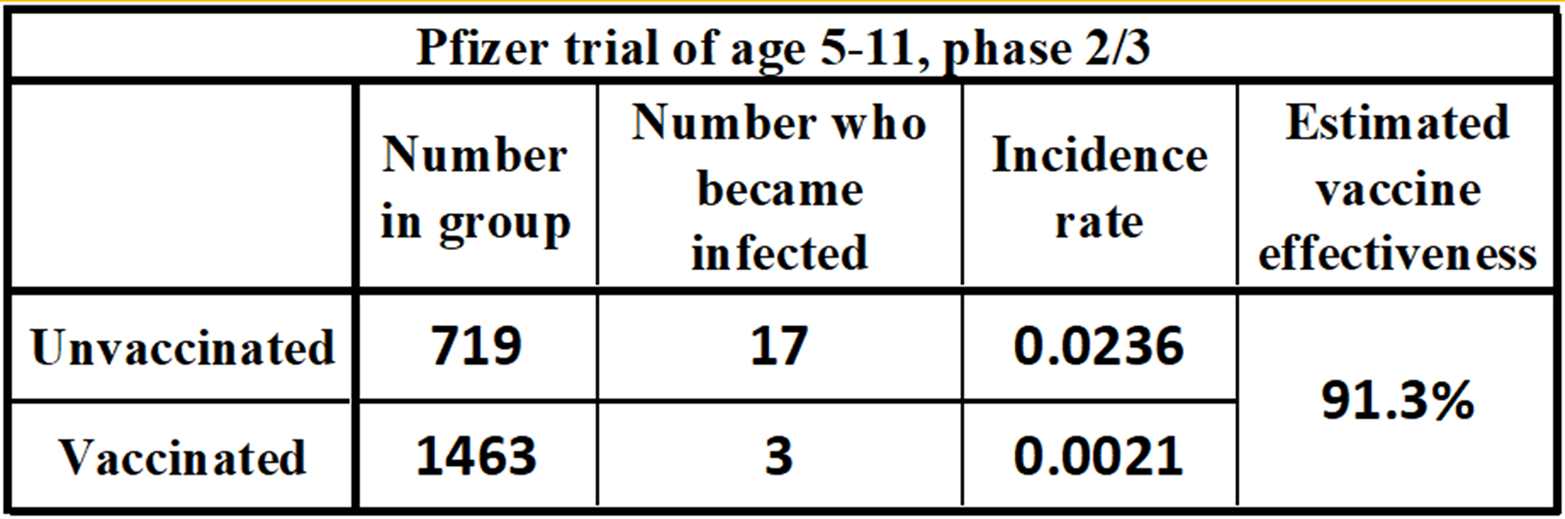
The vaccine effectiveness of the Pfizer COVID trial for children is estimated at: 91.3%.
Immunobridging evidence for effectiveness
There are some cases where it may be difficult or ethically undesirable to conduct large, direct observational studies to prove vaccine effectiveness. Specifically:
- The time element for completing this trial. In exigent situations, immunobridging is a desirable tool.
- The ethics of testing on a subject group (children in this case).
- Any potential reluctance in finding volunteers for the test.
Other relevant test data may then be used to infer the protectiveness of a vaccine. This is called immunobridging, and it often involves the comparison of neutralizing antibody titers between a larger test and the new (smaller) test group. Neutralizing antibody titers are measures of the number of antibodies of the type that fight a particular disease.[7]In some cases, Phase 3 trials may even be skipped through this analysis. This technique has been discussed by officials in various countries around the world in relation to COVID for future vaccine testing.[8] [9] [10]
The immunobridging by Pfizer took blood tests from a subset of the study (322 vaccinated and 163 unvaccinated) and analysed their neutralizing antibodies. The titers from the children’s study were compared to the titers of the adult studies. This showed similar vaccination antibody response between age groups.[11]
This inductive technique has been used for less direct comparisons than between age groups in humans. During the recent Ebola outbreak in Africa, immunobridging was used between non-human primates and humans.[12]
This use of immunobridging increases confidence in the effectiveness of the vaccine, but it does not address the effect of the small study size on understanding adverse effects.
Adverse effects
Pfizer reported no severe adverse effects attributable to the vaccine. There were non-severe adverse effects, which included muscle soreness, fatigue, headache, and chills. There were no reported cases of myocarditis through the study period, which went to three months following the second dose.[13]The small sample size would make a myocarditis event unlikely if rates of occurrence were similar to a nationwide data study in Israel.[14]
Understanding adverse effects and the relative value of vaccination risk versus risk of the disease is important in considering the value of vaccinating, as discussed in this article, Prisoner’s dilemma and vaccination. Evaluating adverse effects is also complex. Researchers must determine whether an adverse effect was caused by the vaccine or not, they must consider sequela (abnormal condition resulting from previous disease), and they must understand expected rates of other disease occurrence in an unvaccinated population.[15]
No member of this study was reported to have experienced severe symptoms of COVID. The three vaccinated children who became infected showed a lower number of symptoms than the 17 unvaccinated children who became sick. However, the small sample sizes of the infected make a comparison of symptoms meaningless.
The scale of the COVID problem for children
We compiled data from the Centers for Disease Control and Prevention (CDC) and the U.S. Census Bureau to catalogue the age-categorized number of reported cases of COVID and related deaths in the United States to Nov. 17, 2021. That data is displayed in the table below.[16][17][18][19]These estimates cannot reflect unreported cases, which are necessarily unknown. Moreover, a material number of reported cases do not include the age of the infected individual and cannot be included. As of Nov. 17, there were 47,471,270 total confirmed cases and 768,619 deaths, but this demographic table only includes 38,503,315 cases and 765,319 deaths.[20]The key points from this data:
- Children contract COVID in significant numbers (> 2 million to date).
- Children die of COVID in lower numbers than adults (104 in the U.S. to date of the 5-11 age group). The survival rate for children who have caught COVID is currently 99.995%
Hospitalization numbers for children are higher than deaths, and are not reported in the chart. As of Oct. 14, 2021, 8,622 children were reported to have been hospitalized due to COVID. About one-third of all hospitalized children did not have any underlying co-morbidities, and two-thirds did.[21]
The relationship of comorbidities to COVID deaths in children warrants further study.[22]But the hospitalization ratio is a good proxy for the comorbidity ratio of deaths.
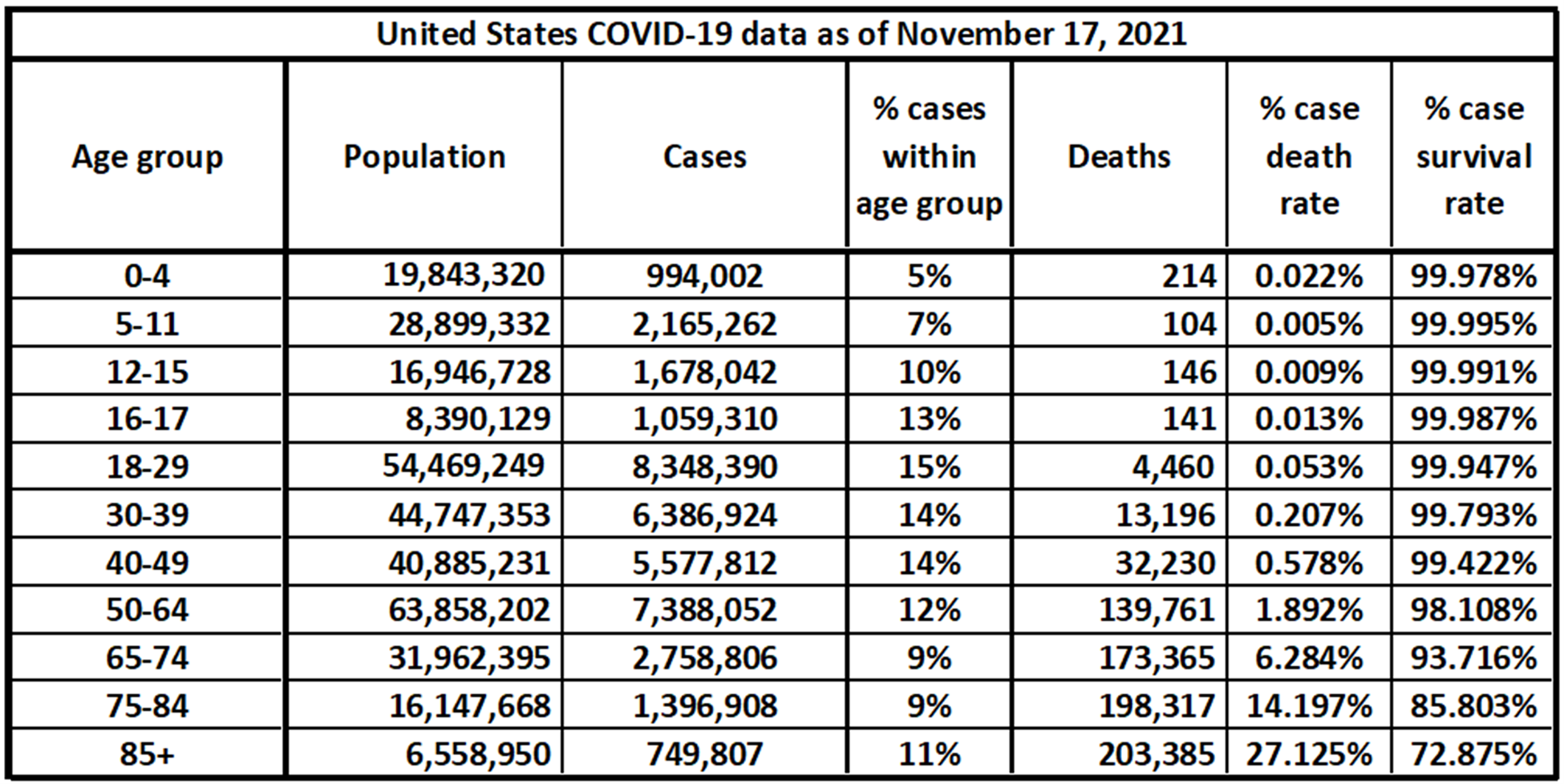
Estimated population in the U.S. by age group, reported cases for each age group, and number of deaths. The percentage of cases within each age group is relative to the population within that age group.[23][24][25][26][27]
Summary
The Pfizer children’s vaccine study showed a similar vaccine effectiveness to the adult studies. A much smaller number of participants were used, though this was somewhat addressed using immunobridging. No serious adverse vaccine effects (there were some mild adverse effects) or severe sickness due to COVID were reported.
Children are infected by COVID in significant numbers, though the proportion of deaths amongst this age group is much lower than for every other age group.
The statistically small risk of severe outcomes (to date) among young children who contract COVID-19 should be at the forefront in any policy-related discussion of vaccination in this age group. The biggest objective concern in regard to safety and adverse events for children are the small sample size and study time in the Pfizer trial. Although no significant adverse events were reported, this should be studied further.
References
[1] U.S. Food and Drug Administration, October 29, 2021, FDA Authorizes Pfizer-BioNTech COVID-19 Vaccine for Emergency Use in Children 5 through 11 Years of Age
[2] Young, Leslie, November 19, 2021, Health Canada approves Pfizer COVID-19 vaccine for children aged 5-11
[3] Roosendaal, Ramon, et al, 2020, Nonhuman primate to human immunobridging to infer the protective effect of an Ebola virus vaccine candidate, Nature Partner Journals Vaccines Vol 5, 112
[4] FDA VRBPAC Briefing Document, October 26, 2021, Pfizer BNT162B2
[5] Polack, Fernando et al, 2020, Safety and Efficacy of the BNT1622 mRNA Covid-19 Vaccine, New England Journal of Medicine, 2020, 383:2603-2615
[6] Polack, Fernando et al, 2020, Safety and Efficacy of the BNT1622 mRNA Covid-19 Vaccine, New England Journal of Medicine, 2020, 383:2603-2615
[7] Roosendaal, Ramon, et al, 2020, Nonhuman primate to human immunobridging to infer the protective effect of an Ebola virus vaccine candidate, Nature Partner Journals Vaccines Vol 5, 112
[8] Balfour, Hannah, September 16, 2021, Immuno-bridging studies are sufficient for authorizing new COVID-19 vaccines, say regulators, European Pharmaceutical Review
[9] Barda, Noam, et al, 2021, Safety of the BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting, New England Journal of Medicine, 385: 1078-1090, DOI: 10.1056/NEJMoa2110475
[10] Liang-yi, Wu, and Kayleigh Madjar, June 18, 2021, Immunobridging still feasible, FDA says, Tapai Times
[11] FDA VRBPAC Briefing Document, October 26, 2021, Pfizer BNT162B2
[12] Roosendaal, Ramon, et al, 2020, Nonhuman primate to human immunobridging to infer the protective effect of an Ebola virus vaccine candidate, Nature Partner Journals Vaccines Vol 5, 112
[13] FDA VRBPAC Briefing Document, October 26, 2021, Pfizer BNT162B2
[14] Barda, Noam, et al, 2021, Safety of the BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting, New England Journal of Medicine, 385: 1078-1090, DOI: 10.1056/NEJMoa2110475
[15] FDA VRBPAC Briefing Document, October 26, 2021, Pfizer BNT162B2
[16] CDC, Nov 17. 2021, Provisional COVID-19 Deaths: Focus on Ages 0-18
[17] CDC, Nov 17, 2021, Provisional COVID-19 Death Counts by Age in Years, 2020-2021
[18] Statista, November 18, 2021, Total number of cases of COVID-19 in the United States as of November 18, 2021, by age group
[19] U.S. Census Bureau, Nov 20, 2021, Population Clock
[20] CDC, Nov 17, 2021, COVID Data Tracker
[21] FDA VRBPAC Briefing Document, October 26, 2021, Pfizer BNT162B2
[22] Makary, Marty, July 19, 2021, The Flimsy Evidence Behind the CDC’s Push to Vaccinate Children, WSJ
[23] CDC, Nov 17. 2021, Provisional COVID-19 Deaths: Focus on Ages 0-18
[24] CDC, Nov 17, 2021, Provisional COVID-19 Death Counts by Age in Years, 2020-2021
[25] Statista, November 18, 2021, Total number of cases of COVID-19 in the United States as of November 18, 2021, by age group
[26] U.S. Census Bureau, Nov 20, 2021, Population Clock
[27] CDC, Nov 17, 2021, COVID Data Tracker
(Lee Hunt – BIG Media Ltd., 2021)



Thank you for your comments, David.
1. Yes, I believe that you are correct.
2. Also a good point. I do plan to follow up with an article (or series of articles) on adverse event reporting and analysis.
You aren’t being cantankerous, btw. I enjoy researching and writing these articles, and I will admit that deciding which points to emphasize and how to do so fairly and objectively can be challenging. And I sometimes struggle a little with how granular to get in the reporting. I often want to write a longer article, but there is the question of what readers will tolerate. Your interest (and expertise on) some of the important details is quite encouraging towards a greater level of detail. We may achieve this through breaking longer pieces into parts.
For your interest, we had a fairly illuminating time editing this particular article. It almost did not get written, we were so concerned about it. And we have another short article coming out in a few days about that process, though our issues were still fairly high level (rather than granular).
Hi, Lee;
excellent article! on your point regarding article length and granularity, I find I definitely enjoy the slightly more concise articles over the longer ones. Perhaps it’s just me, but I think members society in general are developing extremely short attention spans, for various reasons.
Hmm…maybe it’s just me…squirrel!
Hi Lee. There are two important points in this article that, in my opinion, need to be emphasised:
1. Your (and Pfizer’s) estimate of VE is the relative reduction against SYMPTOMATIC infection, not infection as a whole (as the 2nd column in your first table indicates). As far as I can tell, Pfizer did not determine the extent of asymptomatic infection amongst the study’s participants.
2. That ZERO severe COVID-related outcomes (i.e., hospitalisation, multi-system inflammatory syndrome, or death) occurred in either the vaccine or placebo groups over the study period. Maybe this is something that will be addressed in a later study. But, given that Pfizer mentioned this as an “unmet medical need” (page 12 in reference 1), I’m surprised they didn’t plan for that in their trial protocols.
For the first point, I realise I’m being overly meticulous (perhaps cantankerous), but as an epidemiologist, I feel that these distinctions have been blurred throughout the pandemic. And for the second point, I know that you mentioned “severe outcomes” in the article, but I feel its’ “punch”—especially for parents—might’ve been lost coming at the end of the “Adverse Effects” section.
Rant: over. ;D