In an article published by BIG Media Ltd. earlier this year, Lee Hunt presented an analysis of severe COVID-19 outcomes in children based on data from the U.S. Center for Disease Control and Prevention (CDC).[1]
At the time of writing, both Pfizer and Moderna were seeking approval for their mRNA COVID vaccines for children under the age of 5 years, worldwide. That request was granted for the U.S. in mid-June 2022.[2]
Since then, publicly available data on adverse events, or vaccine effectiveness, in children aged under age 5 has received very little attention. This article provides an update on recent findings.
Comparative safety of Pfizer’s mRNA COVID-19 vaccine in German children under age 5
Children younger than 5 years old were more likely to have severe symptoms following Pfizer’s mRNA (BNT162b2) vaccine than after other “routine” vaccines (e.g., influenza, measles, mumps, and rubella, and human papillomavirus), German researchers reported on Oct. 18.[3]
While Germany has not formally approved the mRNA vaccines for young children, it does allow physicians to use them “off label” if parents provide consent to do so.
In their study, Toepfner et al., contacted approximately 19,000 parents and guardians through national vaccination registration databases, resulting in enrolment of 7,806 children in the study April 14 through May 9, 2022.
Compared to approved routine vaccines, Pfizer’s mRNA version was associated with more frequent symptoms related to injection site, musculoskeletal, skin, or mouth and throat pain, but fewer general symptoms – such as fever and malaise – after vaccination.
The frequency with which symptoms were observed increased with mRNA doses above 3 micrograms per injection.
Symptoms reported among the mRNA vaccine recipients were more often persistent beyond the study’s follow-up period. However, Toepfner et al. were unable to determine the severity of those symptoms.
Of those 7,806 children who received mRNA shots, 10 (0.13%) were hospitalized afterward, and 41 (0.53%) required medical attention.
The most surprising aspect of the study is that Toepfner et al. state conclusions that are the opposite of what their data indicate.
For instance, they state in the paper’s “Key Points” that:
“In this study, the overall frequency of adverse events after vaccination with BNT162b2 was comparable with the frequency of adverse events after vaccination with approved non-SARS-CoV-2 vaccines in children younger than 5 years.”
However, looking at outcomes for individual groups of adverse symptoms (see Figure 1), a discrepancy becomes clear.
For example, the odds ratios for musculoskeletal symptoms (e.g., muscle weakness or joint pain) was 2.6 times in-favour of manifesting after the Pfizer vaccines (compared to routine vaccines) but could vary between a 1.3- to 4.9-fold increase. The occurrence of otolaryngological symptoms (such as mouth pain and facial swelling) were more than sextupled but varied from 1.5 to 27.1 times more frequent than other vaccines.
For the authors to observe “comparable” frequencies of adverse events by vaccine type (COVID-19 vs. routine), odds ratios would have to be near, or equal to, 1.
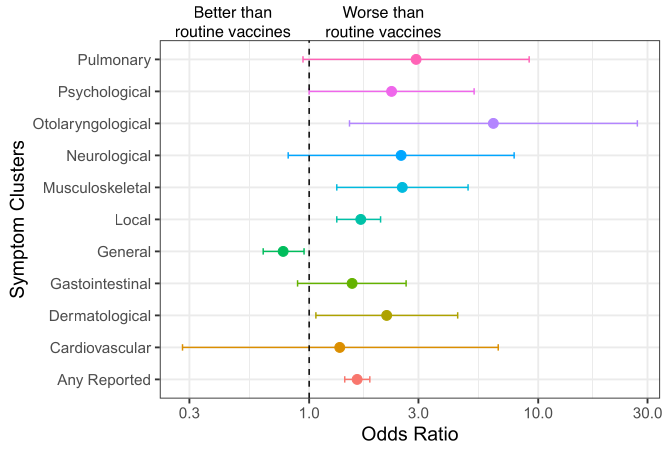
Figure 1: Excerpt from Toepfner et al. displaying symptom clusters occurring after Pfizer’s COVID-19 vaccine vs. other routine vaccinations. An odds ratio > 1 indicates an increase in risk among the mRNA vaccine recipients compared to routine vaccines, whereas an odds ratio < 1 indicates a decrease. The dashed vertical link represents an odds ratio = 1 indicating no difference. Error bars depict lower and upper “limits” of the variability around the estimated odds ratios. Specific examples of symptom clusters are listed in the References & Notes.[4]
Corroborating data from Moderna’s Spikevax trial?
The study by Toepfner et al. is corroborated, in-part, by a report published by the U.S. CDC[5], in which they identified relevant Phase I, II, or III randomized clinical trials of Moderna’s Spikevax COVID-19 vaccine in children aged 6 months to 5 years. Ultimately, the data presented came from a single Phase II/III randomized trial provided by Moderna.
Over a 70-day follow-up, outcomes of interest included individual benefits and harms. The critical benefit of interest was efficacy at prevention of symptomatic and asymptomatic SARS-CoV-2 infection. Data on preventing hospitalization was not available.
The critical harm of interest was serious adverse events (SAEs), such as death or life-threatening event, new hospitalization, or prolonged existing hospitalization, persistent or significant incapacity, or substantial disruption of the ability to conduct normal life functions, or congenital anomalies.
Vaccine reactions were graded on a scale, where grade 3, or greater were deemed important harms. In essence, grade 3 prevents daily routine activity or requires use of a pain reliever, and grade 4 requires emergency room visit or hospitalization.
Compared to the three different dosages (3, 5, or 10 micrograms) studied by Toepfner et al., Moderna’s Spikevax delivers a larger 25-microgram dose of mRNA per injection.
SAEs nearly tripled in vaccine recipients (vs. placebo) but were associated with a wide range of clinically important effect sizes (see Figure 2). One vaccine recipient experienced two SAEs (fever and febrile seizure) that were later determined to be related to the vaccine.
Severe (grade ≥ 3) local or systemic reactions within a week of vaccination were reported by 7.7% of Spikevax recipients, a frequency nearly double that of participants in the placebo cohort (Figure 2), but ranged from a 1.4- to 2.4-fold increase in severe local or systemic reactions.
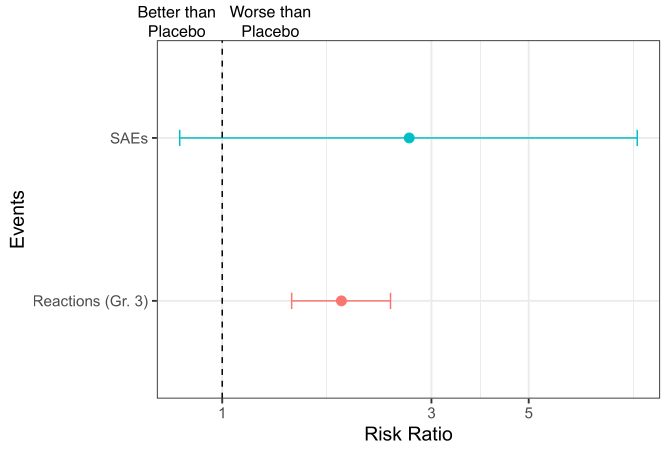
Figure 2. Summary of CDC’s GRADE report on Moderna’s random clinical trial reporting severe adverse events (SAEs) and reactogenicity outcomes (i.e., “expected” adverse reactions) of local and systemic events graded a score of at least 3. As with odds ratios, a risk ratio > 1 indicates an increase in risk among the mRNA vaccine recipients compared to the placebo group and a risk ratio < 1 indicates a decrease. The dashed vertical link represents a risk ratio = 1 indicating no difference. Error bars depict lower and upper “limits” of the variability around the estimated risk ratios.
Lastly, vaccine efficacy (VE) estimates in the 70-day study indicated moderate to low levels of protection against SARS-CoV-2 infections (Figure 3). Estimates resembled many previous VE estimates of seasonal influenza vaccines (e.g.,[6]), ranging from VE = 29% regardless of whether symptoms manifested to VE = 37% for symptomatic infections, and VE = 16% for asymptomatic infections.
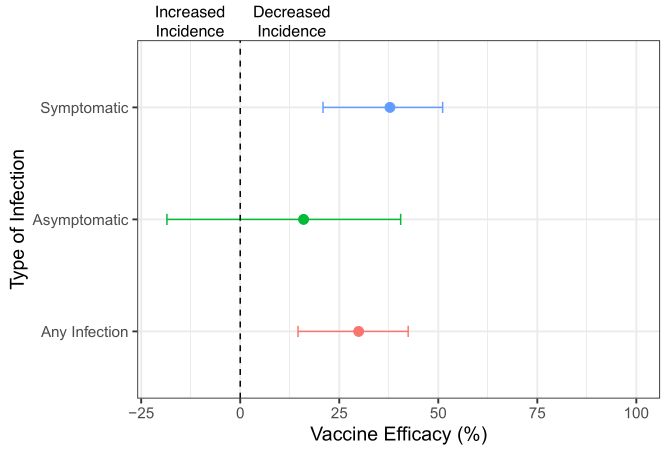
Figure 3. Summary of CDC’s GRADE report on Moderna’s random clinical trial measuring vaccine efficacy over a 70-day follow-up period.
Risk/benefit balance
It is important from a scientific and ethical point of view to scrutinize and discuss as much of the available information as possible, and to make it available to the public.
While we must always consider the age and particularities of an individual child, there remain, at best, unproven benefits, and mounting unnecessary risks. They certainly lend questionable benefit that should, at least, be at the forefront of ongoing research.
Despite some confusing interpretations of their data, Toepfner et al. and the CDC’s report add to the little-noticed safety data from the use of mRNA vaccines in children younger than 5 years old. As shown, the mRNA vaccination offers little benefit to preventing infection, symptomatic or not. Both these clinical trial and real-world findings point to an increase in vaccine-associated safety signals that appear dose-dependent.
In a previous article,[7]BIG Media Ltd discussed higher frequencies of local adverse reactions among adolescent recipients of Pfizer’s mRNA vaccine. When these data were compared to certain (mainly mild) adverse event data of the measles-mumps-rubella vaccine (MMR), adverse events were higher for the mRNA vaccines than for MMR.
Since at least 75% of children in the U.S. have already acquired a natural immunity against COVID-19,[8]and that they are very rarely seriously affected by COVID-19, it begs the question of whether these products have more advantages or disadvantages.
References & Notes
[1] BIG Media: COVID-19’s impact on young children and the question of whether to vaccinate
[2] FDA: Coronavirus (COVID-19) Update: FDA Authorizes Moderna and Pfizer-BioNTech COVID-19 Vaccines for Children Down to 6 Months of Age
[3] Comparative Safety of the BNT162b2 Messenger RNA COVID-19 Vaccine vs Other Approved Vaccines in Children Younger Than 5 Years,Toepfner N, et al. JAMA Network Open 2022; 5(10): e2237140.
[4] The Supplementary Material from Toepfner et al. gives specific signs and symptoms for each broader category displayed in Figure 1. Some examples are listed as follows: Cardiovascular: rapid heart rate, fainting; Dermatological: body rash; Gastrointestinal: abdominal pain, unwanted weight loss; General: fever, malaise; Local: pain, redness at injection site; Musculoskeletal: muscle weakness, joint pain; Neurological: locomotor disorders, dizziness; Otolaryngological: mouth pain, facial swelling; Psychological: mood swings; Pulmonary: shortness of breath, feeling of suffocation.
[5] CDC: Grading of Recommendations, Assessment, Development, and Evaluation (GRADE): Moderna COVID-19 Vaccine for Children Aged 6 Months–5 Years
[6] Savage RD, et al. Euro Surveillance 2015; 20(16): 21100. Link: Strengths and limitations of assessing influenza vaccine effectiveness using routinely collected, passive surveillance data in Ontario, Canada, 2007 to 2012: balancing efficiency versus quality
[7] 7BIG Media: Analysis of COVID vaccine AEFIs, Part I
[8] Most US kids have caught the coronavirus, antibody survey finds, Mallapaty S. Nature 2022; 605(7909): 207.
(David Vickers – BIG Media Ltd., 2022)