The decision to vaccinate a child or not is rationally a question of the risk of the vaccine versus the benefit from the vaccine’s protection against the targeted disease. As reported in the article Prisoner’s dilemma and vaccination, this question can be reframed to the likelihood of infection and the morbidity ratio of the vaccine versus the disease. Information on the morbidity of the disease is often readily available, as in the charts presented in this report Analysis of the Pfizer COVID vaccine trial for children aged 5 to 11, but understanding the morbidity due to the vaccine (an adverse event following immunization, or AEFI) itself is more challenging to determine.[1]
This article takes the first step in outlining the challenge of understanding vaccine morbidity, or AEFIs. We ask the question, “Can we determine what caused an AEFI in an individual case?”
Primary motivation – positive effects of vaccination
There has been a remarkable decline in morbidity and deaths from vaccine-preventable diseases in the 20th and 21st centuries.[2][3]Statistics from the United States regarding a number of these childhood diseases are given in the table below. Vaccination rates greater than 90% are sufficient to reverse outbreaks of most of these diseases, as shown in this article, Examining the effectiveness of restrictions and the path to COVID eradication.
As the chart shows, vaccination rates have dropped since 1998. This is concerning to many immunologists. The issue is that vaccination rates could drop to a low enough rate that some of these diseases could see a resurgence. This is thought to be a result of a public not appreciating the risk of a disease they have never seen (because it has been effectively non-existent during their lifetimes) and concerns over vaccine safety.[4]This is one reason why some immunologists and health professionals have worked to better understand AEFIs.
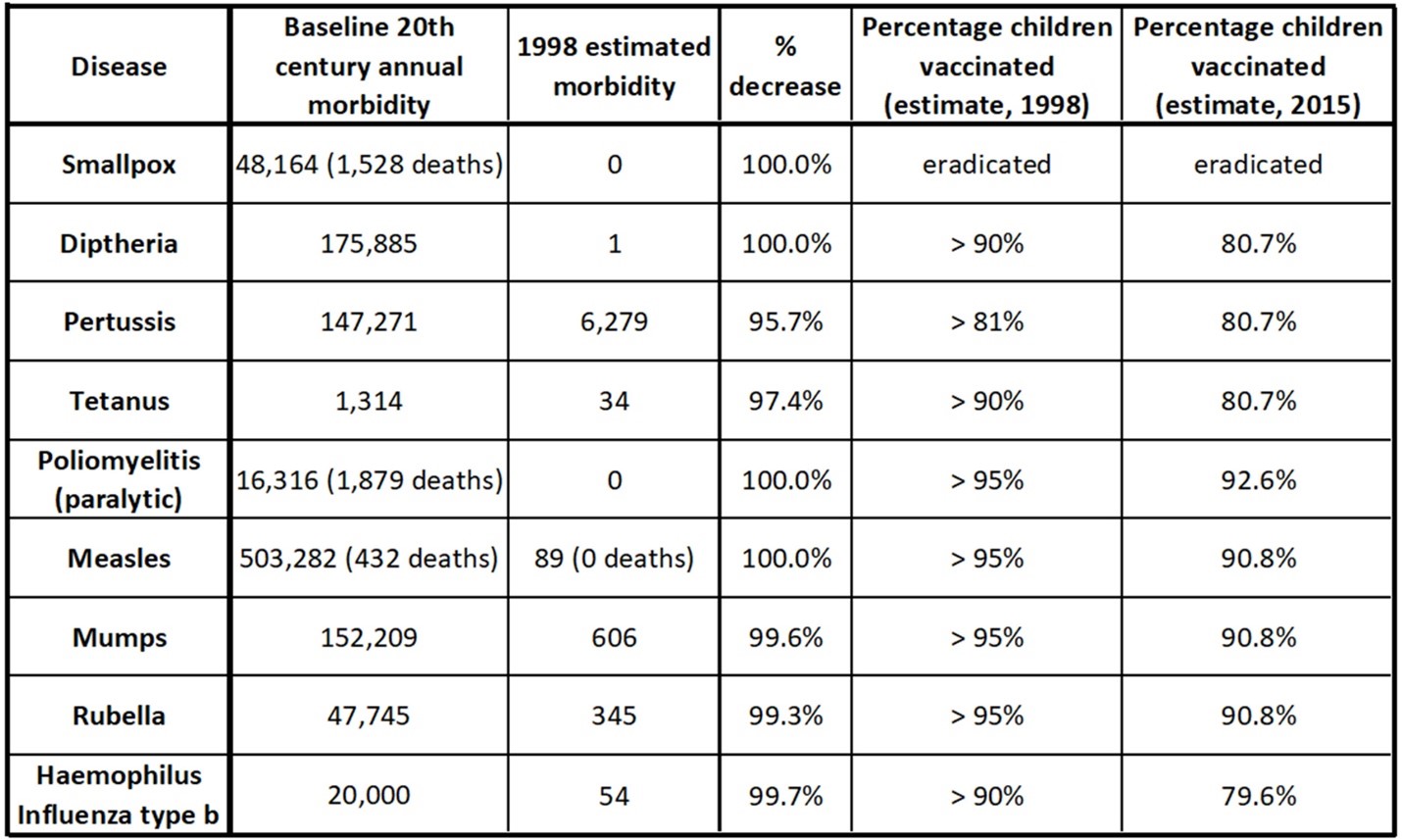
U.S. data for certain childhood diseases and vaccinations in the 20th century. Morbidity can mean cases, or paralysis (in the case of polio). Deaths are reported for polio, smallpox, and measles. Vaccinations rates in the US have dropped since 1998.[5][6]
Secondary motivation – understanding what is behind the troubling headlines
Vaccine trials contend with many factors, but most particularly vaccine effectiveness and vaccine safety. This was outlined in the article Analysis of the Pfizer COVID vaccine trial for children aged 5 to 11 for the recent Pfizer children’s COVID mRNA vaccine. The vaccine effectiveness question has been a topic on many news headlines. For example, a recent news piece titled, “Document Shows Vaccine Deaths WITHIN FIRST 90 DAYS”.[7]This article discusses the original Pfizer mRNA vaccine and reports that “1,200 deaths occurred” in the first three months of the vaccine being given emergency approval.
The problem with this dramatic article is that it does not provide analysis on the 1,200 deaths that apparently occurred. It seems to imply that the deaths are due to the vaccine, but does not delve into how this conclusion was determined. Adverse events do not, by themselves, necessarily indicate drug causality.[8]It is important to estimate scientifically if an AEFI is caused by the vaccine or not.
Analysis of the statistics around a large number of AEFIs will be the topic of a follow-up article. We will begin our understanding by considering a single (individual) AEFI. The principles involved in this analysis will help readers understand the complexities of larger statistical studies and to apply critical thinking to news reports on this subject.
What is an AEFI?
An AEFI is any injurious health event that occurs after a vaccination. This could be a minor event such as a sore injection site, which are often not reported – or it could be somewhat more serious like a fever or body aches, or it could be as severe as a stroke or death.
Causes of AEFIs
It is natural to think of the causes of AEFIs to be a simple dichotomy: something inherent to the vaccine, or a coincidence. We spoke about these possibilities for other events in people’s lives in the article The third hand of fate. A diagram of this dichotomy is given below.
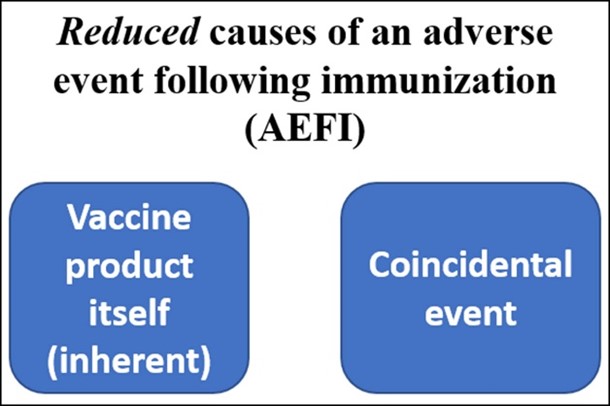
Reduced list of the World Health Organization’s (WHO) potential causes of an AEFI. Reducing our thinking to this dichotomy could result in missing other causal mechanisms for an adverse event.[9]
Life, unfortunately, has greater complexity, making our determination of cause from coincidence more challenging. The WHO published a document on AEFIs with five key categories of causes. This adds manufacturing errors, improper injection, and anxiety as possibilities.
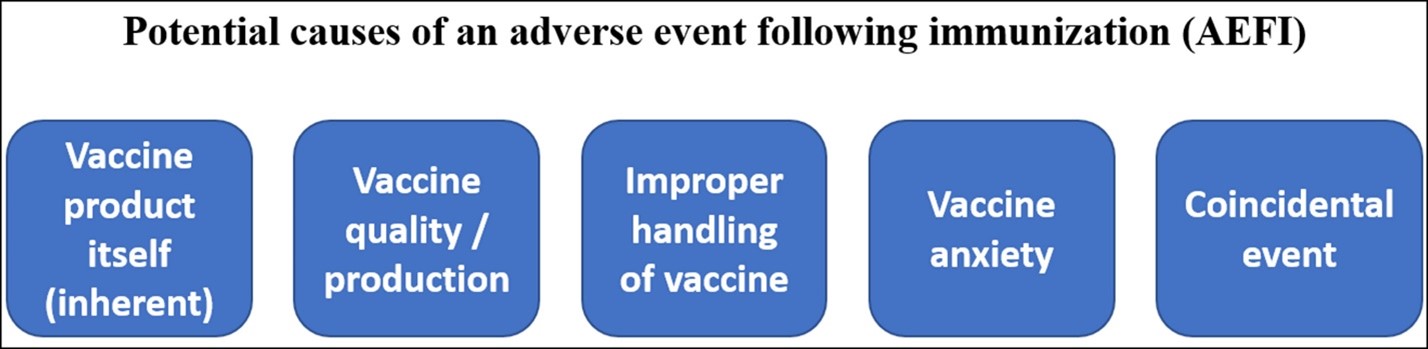
The WHO’s summary of the potential causes of an AEFI. While the focus tends to be on whether an AEFI may or may not be caused by an inherent quality of the vaccine itself, there are other potential causes.[10]
An algorithm to try to determine the cause of an individual AEFI
In many cases, a single, individual AEFI report cannot by itself determine with confidence the cause of the event.[11]A comprehensive reporting of numerous AEFIs is often necessary in undertaking the question of causality.[12]However, this does not mean that such an individual report will always fail to suggest a cause. Furthermore, careful and systematic reporting of AEFIs is essential in the statistical analysis of a large number of reports. So, while we eventually want to talk about statistics from many AEFIs, we first need to understand what we might learn from a single case. This is why various systems exist around the world to properly record such events. The WHO has such a system, which consists of an extensive checklist.[13]In the U.S., healthcare providers have a legal obligation to report AEFIs to the Vaccine Adverse Event Reporting System (VAERS).[14][15]
In 2012, Halsey et al created an algorithm to try to determine the causality of an individual AEFI.[16]Other algorithms have been created for this purpose, some specifically for allergic reactions, but the Halsey algorithm provides the detail necessary for this discussion.[17]
The algorithm has been reproduced below. It requires follow-up testing in many cases, and it is an exercise in inductive logic. As discussed in BREAKING NEWS … into rational pieces, inductive reasoning does not give answers with certainty. Only likelihoods may be provided. In the case of this algorithm, the three possible results are:
- Consistent with causal association (to the vaccine)
- Inconsistent with causal association
- Indeterminate
In many cases, the available data will be insufficient to complete the algorithm.[18]
It should be noted that serious adverse events in the U.S. are required to be reported even if the physician (or reporter) does not think the event is caused by the vaccine.[19] This means that an algorithm such as Halsey’s will not eliminate a death report and that high VAERS death numbers do not by themselves lead to conclusions.
The AEFI algorithm we present is complex because it must consider all the possibilities for AEFIs noted by WHO, including complications from a live virus vaccine or even improper injection.
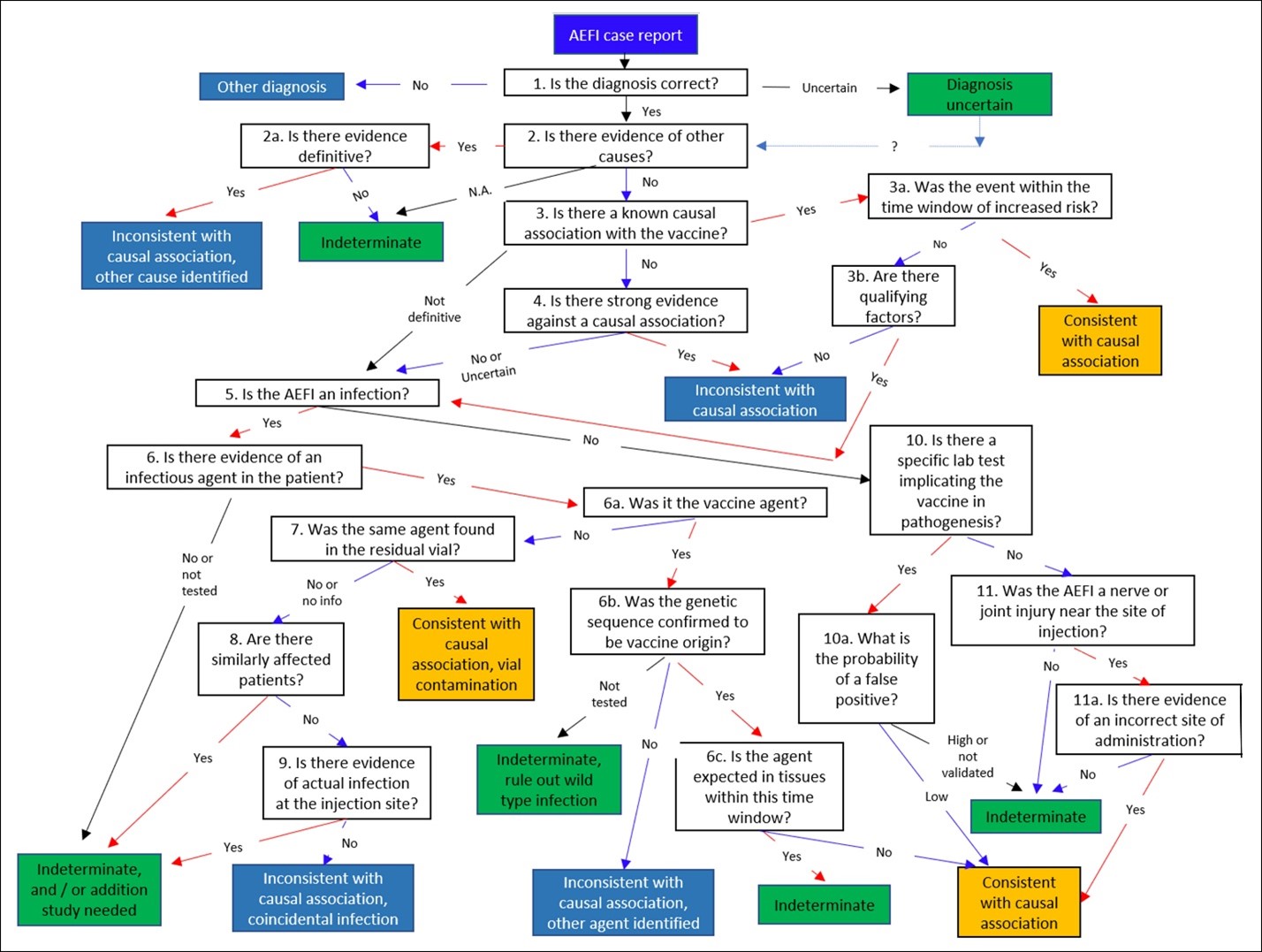
Halsey et al’s 2012 algorithm, which attempts to assess the causality of an individual AEFI report. Most results are not definitive. They can be indeterminate (green) or consistent with a causal association with a vaccine (orange), or inconsistent with a vaccine (blue). Readers should be cautioned that “consistent” or “inconsistent” are not certain. Most individual AEFI reports either lack enough information to give a result other than indeterminate, or are indeterminate regardless.[20]That said, this algorithm is a tool in inductive logic that may be applied.
Why is this so complicated? Time periods, infectious agents, and outcomes
Halsey’s algorithm is so complicated because it must be to provide medical practitioners with a sufficiently rigorous process rooted in what medical research has learned about vaccines, AEFI’s, allergic reactions, and about the vaccine-preventable diseases and other relevant diseases. In order to separate cause from coincidence, an enormous amount of knowledge must be brought to bear.
Most known adverse events have an expected time interval of risk, which is accounted for in the algorithm.[21]For example, an anaphylactic allergic response to a vaccine will normally occur very quickly. Most allergic reactions to vaccines occur within 20 minutes of administration of the vaccine, though a lag of up to four hours is possible.[22]Analysis of AEFIs typically put a time limit (often a day) around an allergic reaction for it to be considered potentially vaccine causal. The time limit must not be left unnecessarily open because other allergic agents become much more likely to be causal at later times.[23]
There is a history of some unintended infections from live-attenuated vaccines. This has included live polio vaccines, to very rare but severe outcomes such as paralysis in about 1 in 760,000 cases.[24]This is why the detection of infection in the patient is critical in certain cases, and is part of the algorithm (steps 5 to 9).
The specific AEFI, or outcome, itself is of importance. Most AEFI studies consider outcomes that have been shown to be relevant in:[25]
(a) Previous vaccine safety studies.
(b) Phase 3 studies of the vaccine in question. This is particularly useful in creating causal associations, but is limited by the study size.
(c) Potential sequalae of the disease being vaccinated against.
(d) Concerns and potential causal associations emerging from surveillance of the vaccine.
This information is vital and is used in the first four steps of the algorithm.
Example: work through the algorithm
The example is: a sore arm at the injection site, the day after injection. The injection was the Pfizer mRNA COVID vaccine for children. Let us work through the algorithm.
- Is the diagnosis correct? Yes.
- Is there evidence of other causes? No.
- Is there a known causal association with the vaccine? Yes.
3a. Was the event within the time window of increased risk? Yes.
Result: consistent with a causal association. Which mean, perhaps.
Example 2: Encephalitis (actual example)
A 35-year woman old received an intranasal, live-attenuated influenza vaccine. She developed numerous symptoms (sore throat, body aches, fever, chest pain) two days later. This worsened into a severe headache. Chest X-ray showed no pneumonia. 11 days after vaccination, she was admitted to an emergency room with confusion and vomiting. She was given a cerebrospinal fluid (CSF) test, and viral cultures from the CSF revealed an enterovirus. The woman was treated for the virus, and her disease was resolved within three days. Let us work through the algorithm:
- Is the diagnosis correct? Yes.
- Is there evidence of other causes? Yes. The enterovirus, not an influenza.
2a. Was the event within the time window of increased risk? Yes.
Result: inconsistent with causal classification, other cause identified. The woman had coincidentally caught a virus that causes encephalitis. However, if the viral cultures had not been taken from the CSF, the conclusion may erroneously have been either “indeterminate” or “consistent with causal association.”
Interpretation – where do we go from here?
It is important to know if an adverse event following immunization (AEFI) is caused by a vaccine. This speaks to vaccine safety and is an essential element in the decision to vaccinate or not. A single AEFI, or an accumulation of adverse events, would not, in isolation, address the question of vaccine safety. To determine if the event is causal or coincidental, testing and inductive logic are necessary.
The single AEFI causal algorithm shown in this article illustrates how difficult it is to:
(a) Determine what the likely causal mechanism of the AEFI is, whether it is vaccine or other.
(b) Have the information necessary to complete the algorithm. As demonstrated, significant testing is required in order to complete the algorithm.
(c) Have certainty. In most cases, the algorithm can only speak to whether the AEFI is “consistent”, “inconsistent”, or “indeterminate”.
In the article The third hand of fate, we talked about causes and coincidences. We showed that knowledge of base rates is helpful in separating causes from coincidences. This will come into use in our next article. There, we will use the knowledge from the individual AEFI algorithm and combine it with information in base rates and statistics for a large number of AEFIs. This approach will lead to a more complete assessment of vaccine safety.
References
[1] Halsey, Neal, et al, 2012, Algorithm to assess the causality after individual adverse events following immunizations, Vaccine, V 30 5791-5798
[2] National Immunization Program, CDC, 1999, Achievements in public health 1900-1999: impact of vaccines universally recommended for children—United States, 1900-1998, MMWR Morbidity and Mortality Weekly Report 1999; 48, 243-249
[3] National Center for Health Statistics, CDC, 2019, Immunization
[4] Halsey, Neal, et al, 2012, Algorithm to assess the causality after individual adverse events following immunizations, Vaccine, V 30 5791-5798
[5] National Immunization Program, CDC, 1999, Achievements in public health 1900-1999: impact of vaccines universally recommended for children—United States, 1900-1998, MMWR Morbidity and Mortality Weekly Report 1999; 48, 243-249
[6] National Center for Health Statistics, CDC, 2019, Immunization
[7] Nukta, December 12, 2021, Document Shows Vaccine Deaths WITHIN FIRST 90 DAYS, Nukta LiveJournal
[8] FDA, 2021, 5.3.6 Cumulative Analysis of POST-Authorization Adverse Event Reports of PF-07302048 (BTN162B2) Received Through 28-Feb-2021
[9] World Health Organization, 2021,Causality assessment of an adverse event following immunization (AEFI): user manual for the revised WHO classification, 2nd ed., 2019 update. Geneva: WHO, 2021
[10] World Health Organization, 2021,Causality assessment of an adverse event following immunization (AEFI): user manual for the revised WHO classification, 2nd ed., 2019 update. Geneva: WHO, 2021
[11] Halsey, Neal, et al, 2012, Algorithm to assess the causality after individual adverse events following immunizations, Vaccine, V 30 5791-5798
[12] Halsey, Neal, 2002, The Science of Evaluation of Adverse Events Associated with Vaccination, Seminars in Pediatric Infectious Diseases, V 13, N 3, 205-214
[13] World Health Organization, 2021,Causality assessment of an adverse event following immunization (AEFI): user manual for the revised WHO classification, 2nd ed., 2019 update.Geneva: WHO, 2021
[14] VAERS, Vaccine Adverse Event Reporting System
[15] Centers for Disease Control and Prevention, downloaded November 21, 2021, Manual for the Surveillance of Vaccine-Preventable Diseases, Chapter 21: Surveillance for Adverse Events Following Immunization Using the Vaccine Adverse Event Reporting System
[16] National Immunization Program, CDC, 1999, Achievements in public health 1900-1999: impact of vaccines universally recommended for children—United States, 1900-1998, MMWR Morbidity and Mortality Weekly Report 1999; 48, 243-249
[17] Roches, Anne, et al, 2021, Evaluation of Adverse Reactions to Vaccines, The Journal of Allergy and Clinical Immunology In Practice, V9 N10, 3584-3597
[18] Halsey, Neal, et al, 2012, Algorithm to assess the causality after individual adverse events following immunizations, Vaccine, V 30 5791-5798
[19] VAERS, Vaccine Adverse Event Reporting System
[20] Halsey, Neal, et al, 2012, Algorithm to assess the causality after individual adverse events following immunizations, Vaccine, V 30 5791-5798
[21] Halsey, Neal, et al, 2012, Algorithm to assess the causality after individual adverse events following immunizations, Vaccine, V 30 5791-5798
[22] Roches, Anne, et al, 2021, Evaluation of Adverse Reactions to Vaccines, The Journal of Allergy and Clinical Immunology In Practice, V9 N10, 3584-3597
[23] Klein, Nicola, et al, 2021, Surveillance for Adverse Events After COVID-19 mRNA Vaccination, JAMA 2021: 326(14) 1390-1399, DOI:10.1001/jama.2021.15072
[24] Halsey, Neal, 2002, The Science of Evaluation of Adverse Events Associated with Vaccination, Seminars in Pediatric Infectious Diseases, V 13, N 3, 205-214
[25] Klein, Nicola, et al, 2021, Surveillance for Adverse Events After COVID-19 mRNA Vaccination, JAMA 2021: 326(14) 1390-1399, DOI:10.1001/jama.2021.15072
(Lee Hunt – BIG Media Ltd., 2021)


