In the article Taking a responsible look at adverse events following immunization, we discussed the difficulty in determining the cause of an individual report of an adverse event following immunization (AEFI). Finding out whether the event was caused by coincidence (something else) or the immunization was shown to be an exercise in inductive logic, one that often requires follow-up examination or information. It is also an exercise that may fail to yield a definitive answer.
In this two-part article, we examine the use of a group of AEFIs in a study to better assess vaccine safety. The vaccines that we examine are mRNA COVID-19 vaccines.
In order to perform this analysis, it helps to first review the article linked above, and The third hand of fate, where we discussed the use of statistics to separate cause from coincidence.
We will look at three studies:
- A cohort study of the adverse events recorded on the Pfizer Phase 3 analysis for 12–15-year-old adolescents compared to 16–25-year-old participants from an older study. 2,260 adolescents were enrolled in this study.[1]
- An interim report based on surveillance data in the United States. This involved eight participating health care institutions and 6.2 million vaccinated individuals within their care plans.[2]
- Three nationwide studies performed in Israel.[3][4][5]
Each study involves the determination of base rates of adverse events with an experimental design meant to provide control so that these base rates may be compared validly. Base rates may refer to some normative background rate for an adverse event in a population, or they may refer to the rate of some other causal event of interest. In this article we use them in two ways:
(a) AEFI of vaccinated individuals compared to a base rate from unvaccinated individuals.
(b) Base rates of outcomes from a disease compared to AEFI rates of vaccinated individuals.
Comparisons of AEFIs and base rates require that the groups of individuals being compared are medically similar. Meaning, they have similar ages, ethnic mixes, general health factors, comorbidities, or other variables that may be deemed medically relevant.
A vetting of the events was also conducted at a study-defined level during the trial (in Study 1) or through the health care institutions involved (in Studies 2 and 3). This vetting would encompass some of the elements in the algorithm discussed for the assessment of individual AEFIs in Taking a responsible look at adverse events following immunization, but would not be as comprehensive.
Special problems inherent in a new type of vaccine
The process of analysis of any AEFI requires knowledge of whether the event could be considered to be consistent with the immunization. As we showed in Taking a responsible look at adverse events following immunization, such information comes from study and history, including the following:[6][7]
(a) Previous vaccine safety studies.
(b) Phase 3 studies of the vaccine in question. This is particularly useful in creating causal associations, but is limited by the study size.
(c) Potential sequelae of the disease being vaccinated against.
(d) Concerns and potential causal associations emerging from surveillance of the vaccine.
This means that the study of COVID vaccines could potentially have three problems:
- Lack of history with the vaccine type, in the case of mRNA vaccines. This speaks to (a) above. We do not know as much about mRNA vaccine safety as we do with vaccine types that have a longer history of use.
- An initial dependence of the Phase 3 studies until a history of mass vaccination is built. This speaks to the importance of (b).
- Lack of history with the disease and its sequelae. This speaks to (c).
This is not to say that we should assume mRNA vaccines cannot be understood, or presume problems with them. It does, however, mean that we must be more careful in our surveillance of new potential causal associations with the vaccine. Humility and diligence are logically required.
Study types matter: cohort studies versus large-scale surveillance
Individual study designs may be suited for identifying specific types of adverse events, and may be unsuited to identifying other types of events. This is well known in medical literature, but is also a logical consequence of the design.[8][9]
For example, Phase 3 vaccine trials typically have a very careful control design over the cohorts. Individuals are chosen during the enrolment phase in a way that minimizes confounding variables. They may also be monitored closely for adverse events, much more so than the general public would be. This means that such a study is much more likely to identify mild adverse events. A large surveillance-style study depends on patients presenting themselves to their doctors and the health-care professional reporting the event. It is self-evident that many mild adverse events will not be reported under such an experimental design. Such an effect is inherent in the self-controlled nature of this type of study.[10]
In quite the opposite way, the surveillance studies have an advantage over the smaller cohort studies for investigating serious events. Most serious adverse events are too infrequent to be caught in a Phase 3 trial, but the larger surveillance studies, which can have millions of individuals, are much more statistically likely to encounter them.[11][12] This is summarized in the table below.
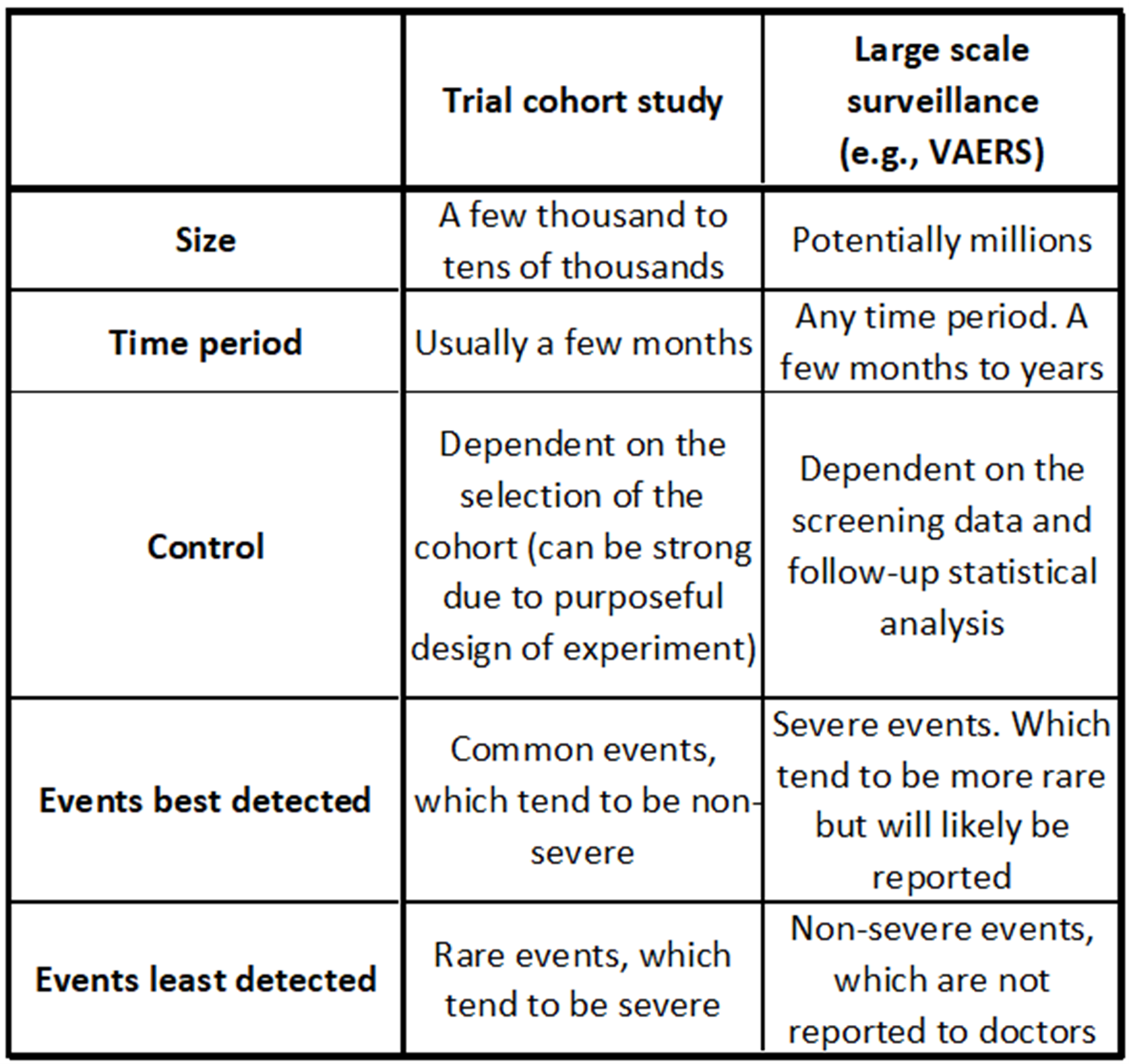
Table illustrating general strengths and weaknesses of cohort studies such as in vaccine trials versus studies resulting from large-scale surveillance. There can be exceptions to these generalizations. Severe events could be common, leading to the rejection of a vaccine, and non-severe events can also be rare. Event detection always depends on the likelihood of an event being reported.[13][14]
Risk ratios – how often do severe complications from vaccines occur?
All of BIG Media’s reporting of COVID vaccine safety to date has been concerned with severe adverse events. This is the main focus of vaccine safety, but it should not be the only focus. We will report on mild events in this article, but it is critical that the most serious adverse events be well understood.
A table below summarizes the historical understanding of serious AEFIs.[15][16][17][18]It includes mortality data from the diseases being vaccinated against, which allows us to create risk ratios (RR) for severe outcomes.[19]The risk ratio is the ratio of the morbidity due to the disease over the morbidity due to the vaccine. In the article Prisoner’s dilemma and vaccination, we showed that a variation of this ratio is critical in the rational decision to vaccinate or not.
Another way to look at risk ratios is to understand that they are a compact method of normalizing adverse events by a chosen base rate. Although the table below compares the risk ratio of the disease versus the vaccine to measure the payoff of vaccinating, risk ratios can be used in other ways. For example, we will later compare the risk ratio of an adverse event for vaccinated versus unvaccinated persons.
In this table, RR is calculated by dividing the disease incidence (death unless otherwise stated) rates by the given severe vaccine complication (AEFI) incidence rate. The higher the RR number in this comparison, the better. However, readers should always compare the absolute value of either the AEFI incidence or disease outcome (how often it occurs) as well as the RR. Both measures are meaningful.
For example, Polio causes permanent paralysis in about 0.5% of cases, but the vaccine causes paralysis in 1/760,000 cases, so the RR of paralysis for the polio disease versus the vaccine is about 3,800. This means that a patient is 3,800 times more likely to develop polio paralysis from polio than from the vaccine for polio.[20]Also, we know that polio causes paralysis in a high absolute sense (0.5% of people who contract the disease). In the chart, we compare death from polio rather than paralysis, so the RR is given at 1,000.
The risk ratio for most severe adverse events is on the order of 1,000.[21]It should be noted that both the vaccine adverse events and disease outcomes may have multiple possibilities that are severe. The specific outcomes being compared must be clearly identified. To illustrate the potential granularity of this problem, we broke out three severe adverse events from the measles vaccines and noted three additional severe outcomes for the disease.
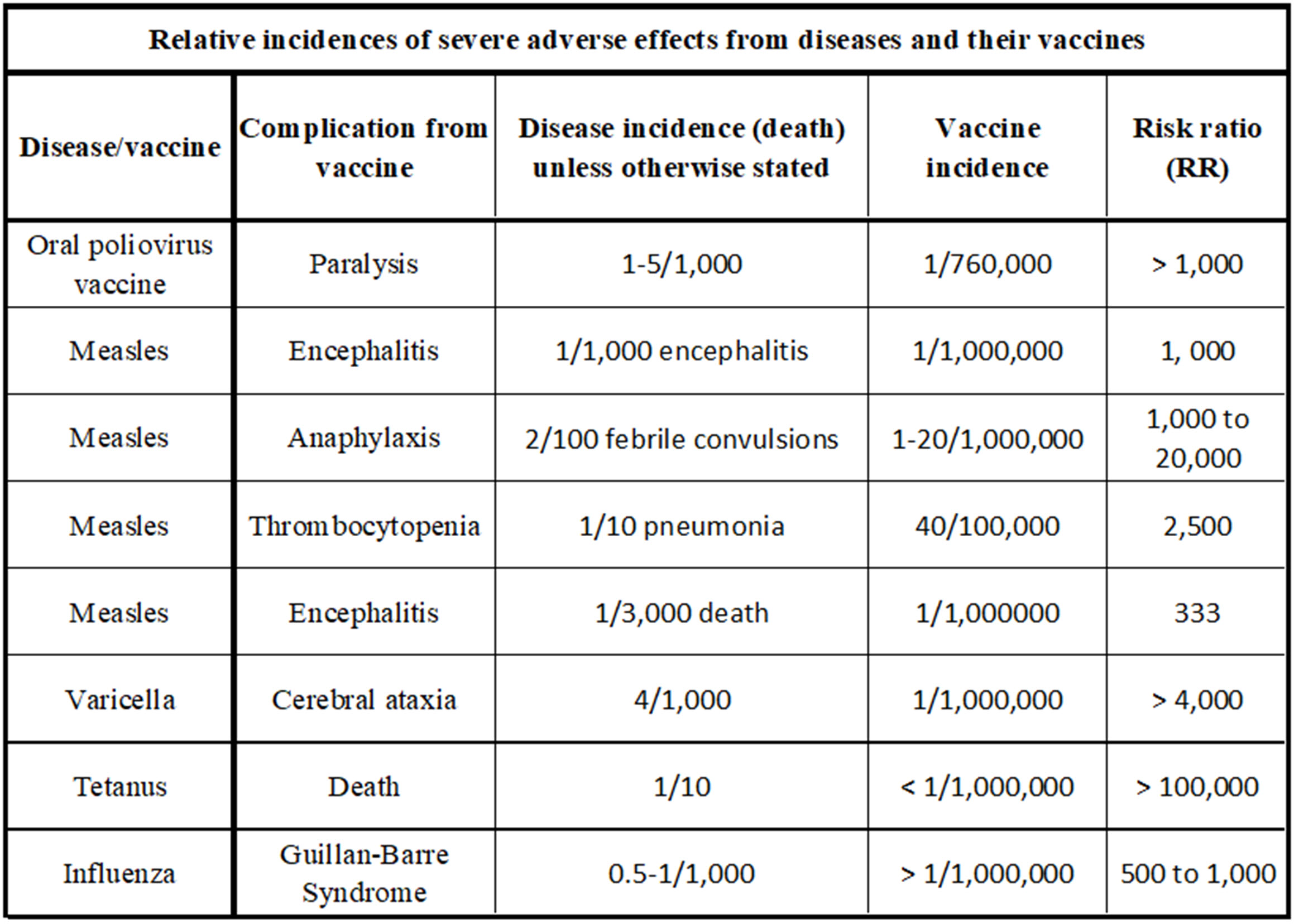
The relative incidence of severe adverse effects from diseases and their vaccines. The disease incidence given in this chart is relative to death unless otherwise stated. Many diseases have multiple severe outcomes, and their vaccines may also have multiple severe outcomes. This is detailed illustratively for measles.[22][23][24][25]Risk ratios in the order of 1,000 are expected for most of these examples.
Study 1: Pfizer’s Phase 3 trial for adolescents
A total of 2,260 participants in the 12–15 age category were split into two groups – 1,131 individuals were in the vaccine group, and 1,129 were in the placebo group. Each group received two injections, 21 days apart. These subjects were monitored for vaccine efficacy and safety. We focus on the safety aspect in this article. The efficacy, however, was 100%.[26]AEFIs for these subjects were recorded.
AEFIs were also noted from the previous (adults) Phase 3 trial for the 16–26 age group and treated to a similar safety analysis.[27]
The results are broken out in the following ways:
- Whether they are local events or systemic events
- By dose number
- By risk ratios (RR). RR values much higher than 1 suggest that the vaccine is causal.
- By percentage, and displayed in both tabular and graphical form.
Evaluating the data
Both the RR values and the absolute incidence rates are important. A high RR and a high incidence rate mean that the AEFI is causal and common.
The tables compare vaccinated groups to corresponding unvaccinated (placebo) groups. An example follows:
In the first table, “Local adverse events, ages 12-15 and 16-25, Dose 1”, note that we are comparing AEFIs from the age categorized vaccinated group to the AEFI incidence rate in the corresponding placebo group. So for “Redness”, 6% of vaccinated those aged 12-15 developed the symptom, and 1% of the placebo group of the same age developed the symptom. And 6/1 = 6. The RR is 6 for children aged 12-15 for Redness.
The other columns (and other tables) follow this same rationale.
Results
No severe AEFIs were noted, and all the symptoms were resolved. High percentages of both local and systemic mild events were found. The risk ratios were high enough that there is little doubt that all the charted AEFI types were vaccine causal, with the possible exception of diarrhea.
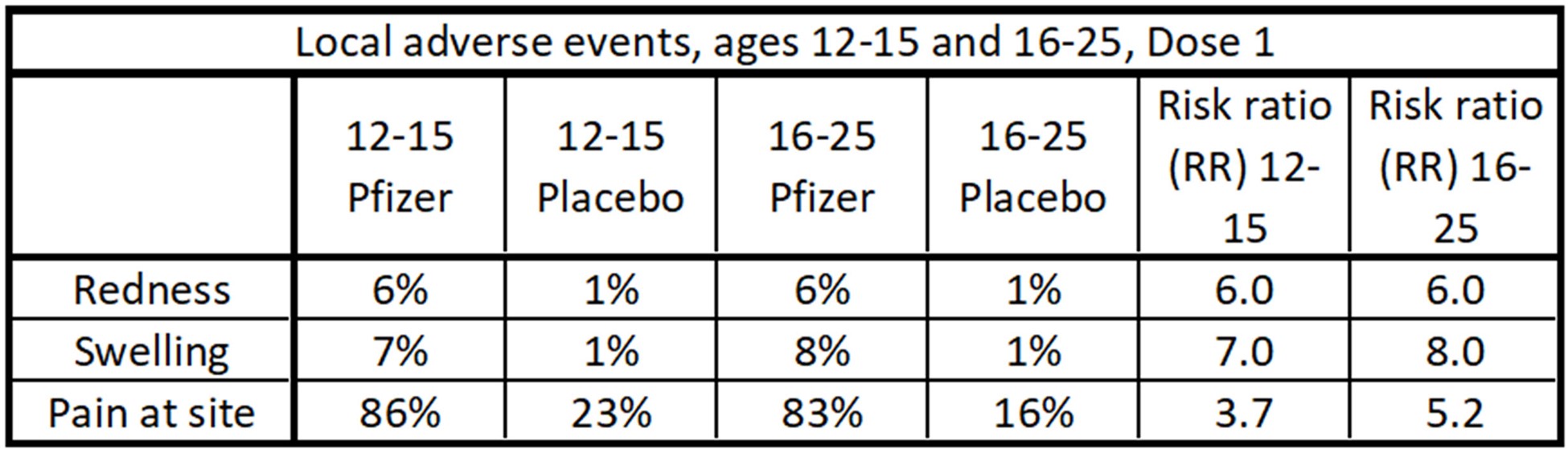
Data table of local adverse events after Dose 1 for the Pfizer study.
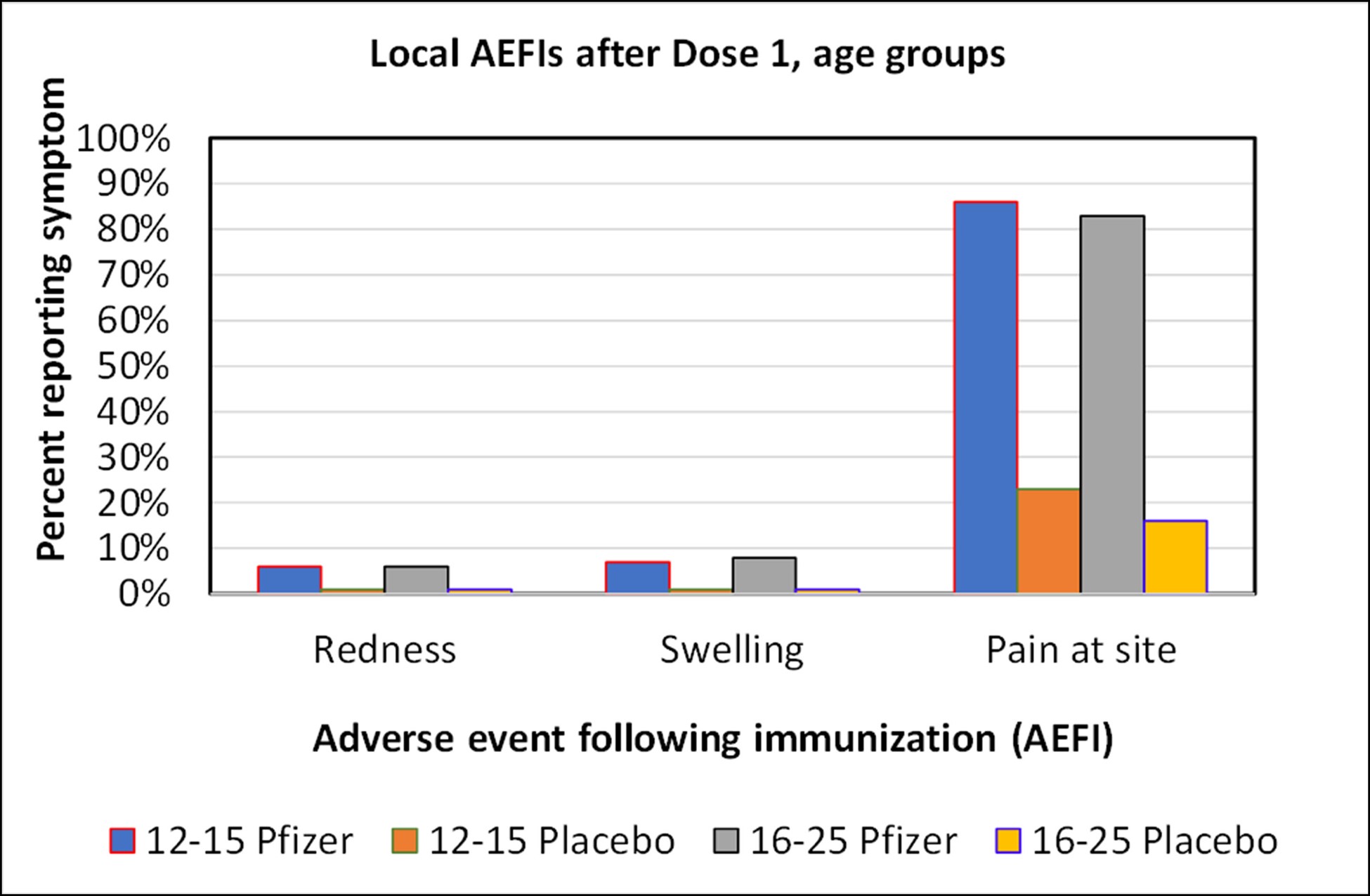
Graph of local adverse events after Dose 1 for the Pfizer study. There was no material difference in the frequency of adverse events between these age groups.
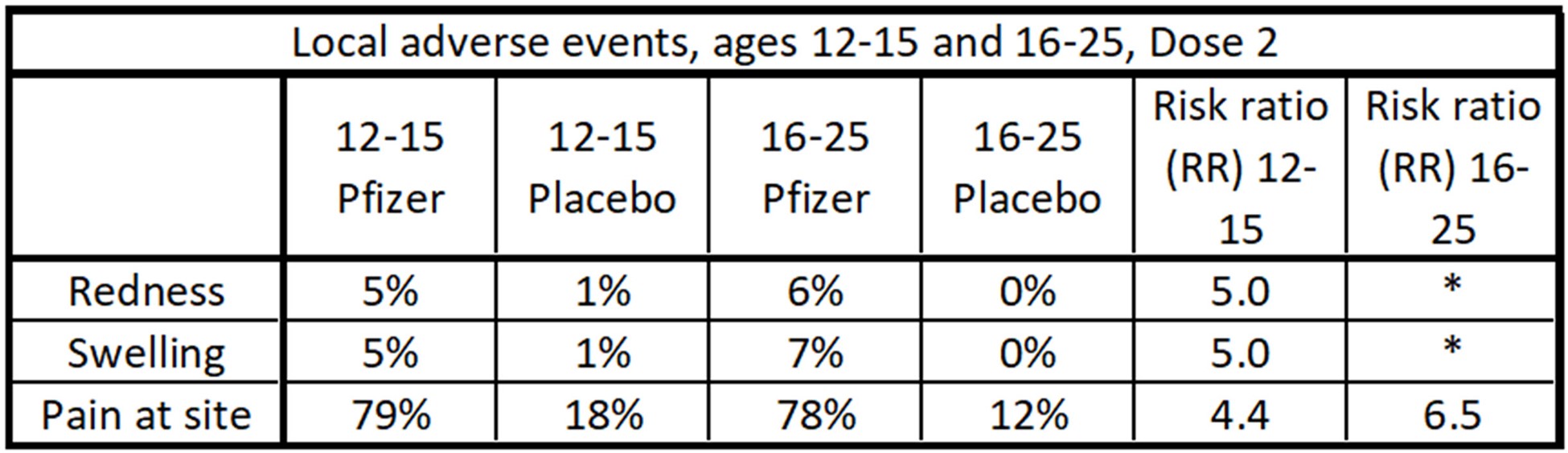
Data table of local adverse events after Dose 2 for the Pfizer study. Pain at the site was a common occurrence, with a risk ratio (RR) of between 4 and 6.5. The ‘*’ indicates an infinite apparent risk ratio, which is a phenomenon of the small number of individuals in the study. Since the absolute percentages of these events were low, this is not a big concern.
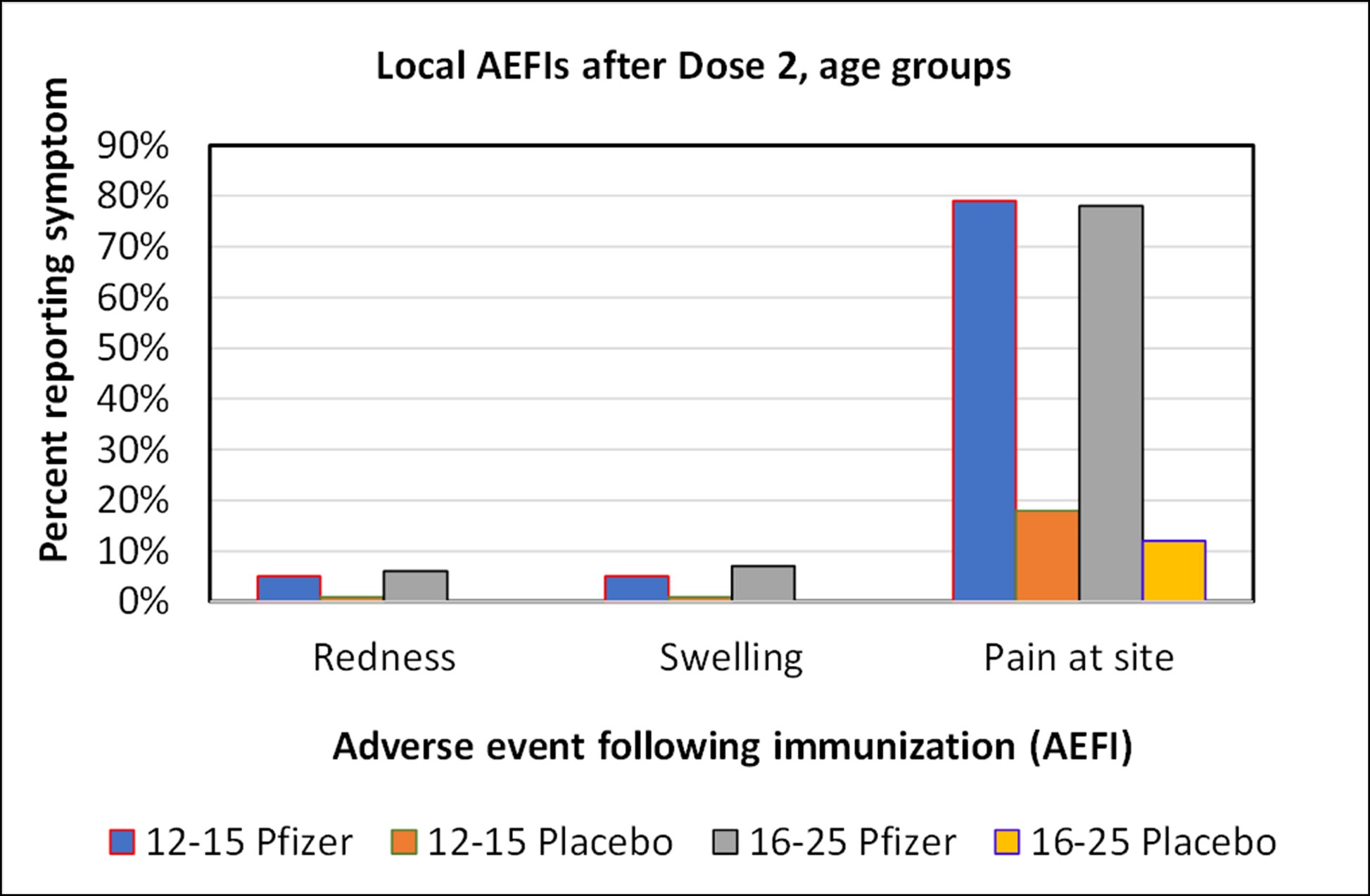
Graph of local adverse events after Dose 2 for the Pfizer study. There was no material difference in the frequency of adverse events between these age groups.
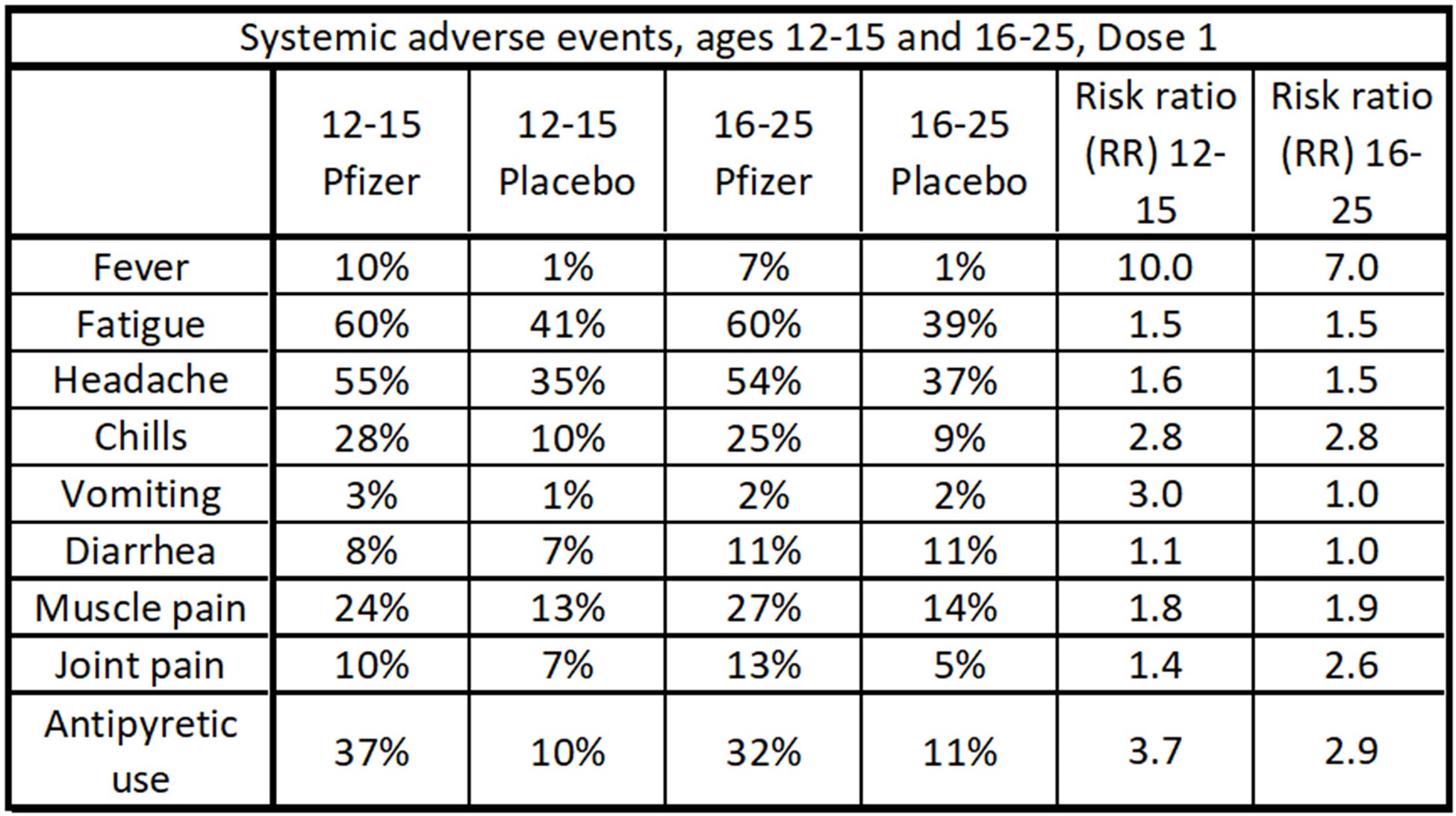
Data table of systemic adverse events after Dose 1 for the Pfizer study. Fatigue and headaches were common occurrences, but the risk ratio was not as high (except for fever) as it was in the local events. Antipyretic drugs such as over-the-counter Tylenol reduce fever symptoms.
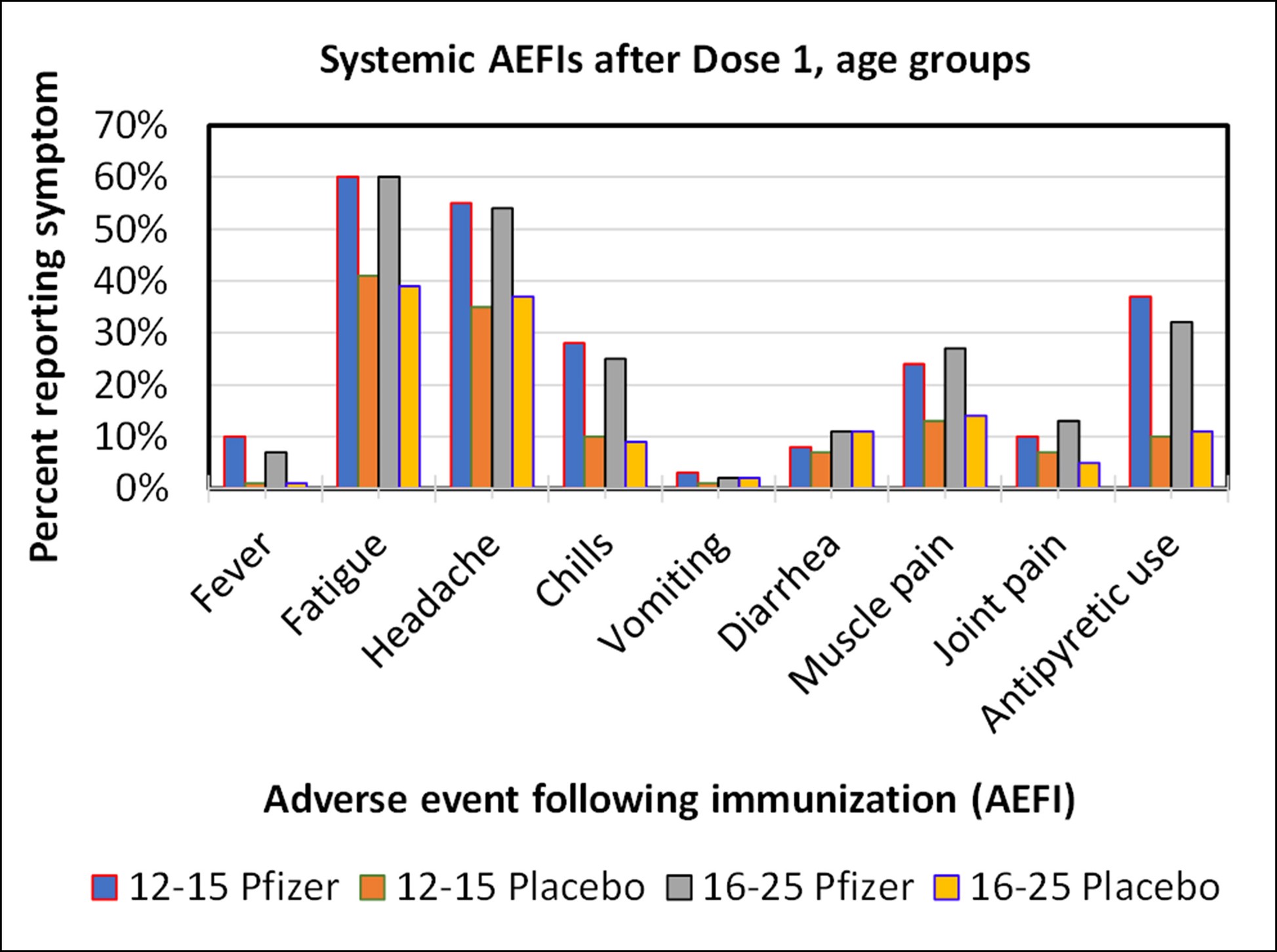
Graph of systemic adverse events after Dose 1 for the Pfizer study. Fatigue and headaches were common occurrences. There was no material difference in the frequency of adverse events between these age groups.
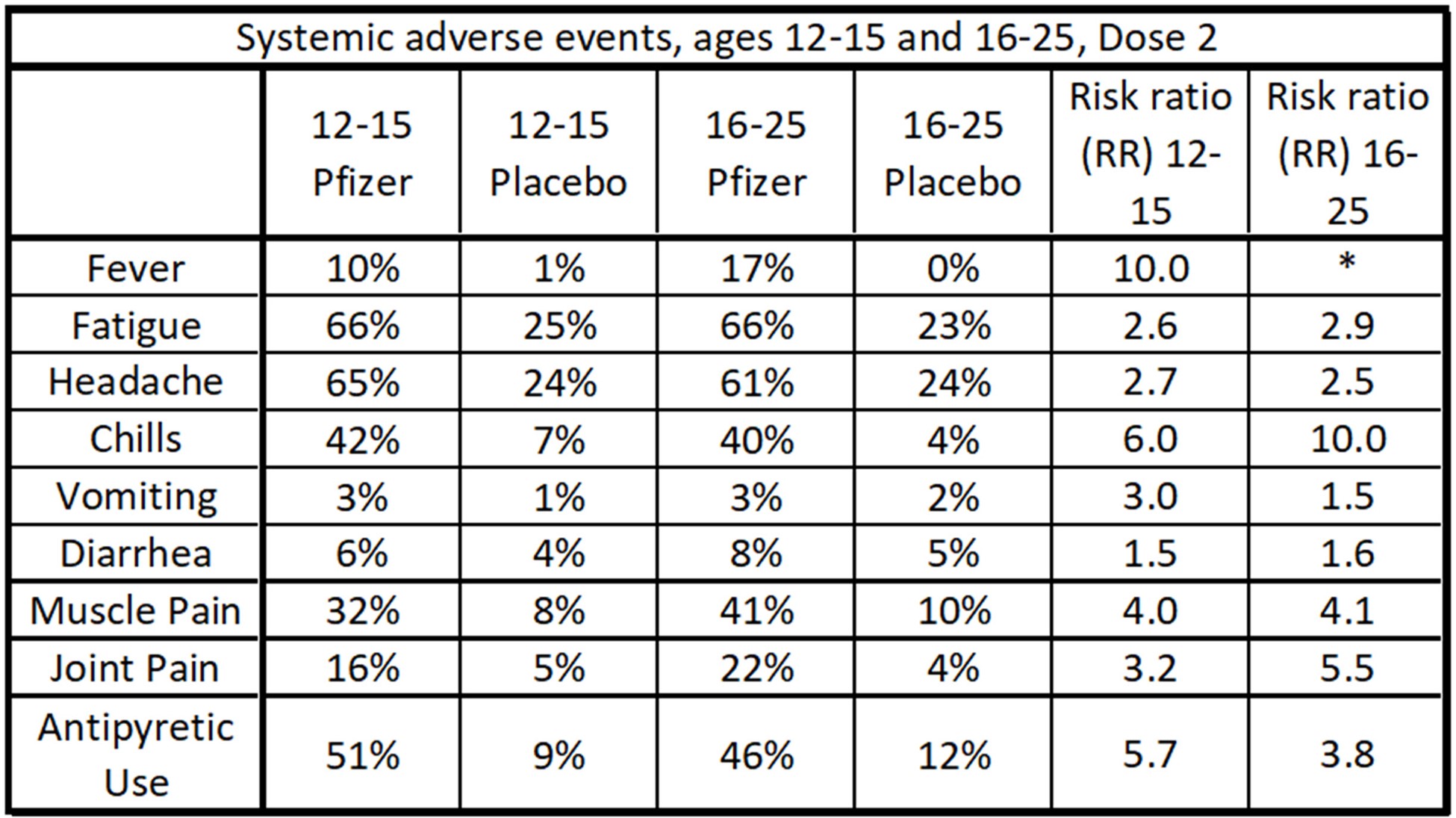
Data table of systemic adverse events after Dose 2 for the Pfizer study. Fatigue, headaches, muscle pain and chills were common occurrences.
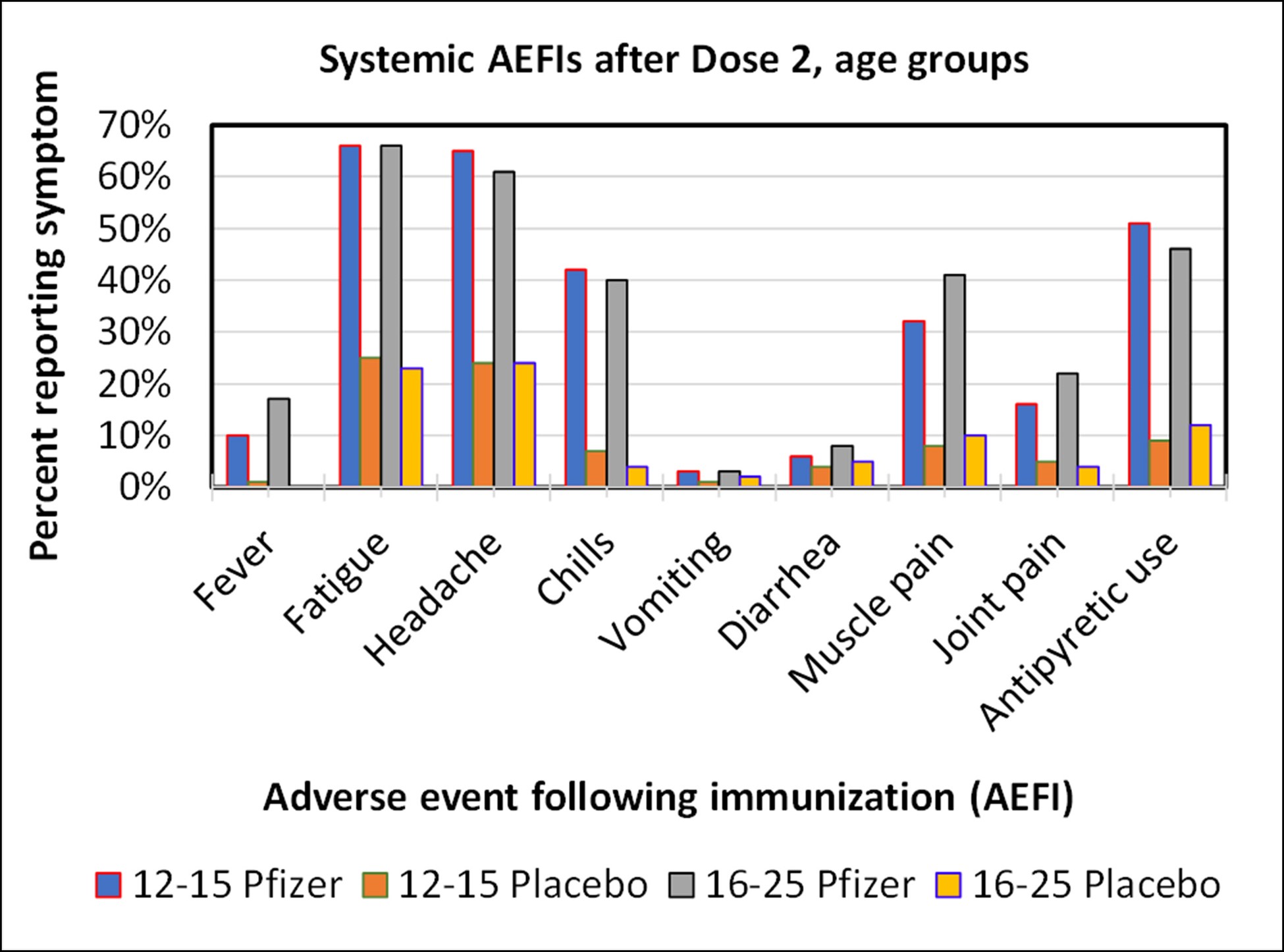
Graph of systemic adverse events after Dose 2 for the Pfizer study. Fatigue, headaches, muscle pain and chills were common occurrences. There was no material difference in the frequency of adverse events between these age groups.
Perspective – a measles, mumps, and rubella (MMR) vaccine cohort study of non-severe AEFIs
Is it of concern that such a high percentage of the subjects experienced mild symptoms? We refer to another study for perspective.
It has been noted that many children given the MMR vaccine showed mild AEFIs, including respiratory symptoms, nausea, vomiting, and fever within the first 21 days of injection.[28]A cohort study was designed to investigate this using 581 carefully selected twin subjects. By “twins” we mean pairs of children of comparable age and other factors designed to minimize confounding variables. One member of the pair is given a placebo, and the other the MMR vaccine. All subjects were monitored for 21 days. This control allows for accurate risk ratio estimates.[29]The results were interesting. Of the four types of AEFI noted, three of them (respiratory symptoms, nausea, vomiting) were more frequent in the placebo group than the vaccinated group. This means that the risk ratio was below 1 for these symptoms. Only one symptom, fever, had higher incidence in the vaccinated group, and that was marginal for this study size. The risk ratio was never higher than 1.3.
What does this suggest?
- Children and adolescents are subject to many diseases and disease symptoms, which can be erroneously attribute to a vaccination. Reference to base rates and risk ratio is essential in drawing conclusions.
- That the MMR vaccine does not seem to cause these particular adverse events, except possibly fever.
- That the COVID mRNA vaccine AEFI results are quite different from the MMR vaccine. The risk ratios from the COVID vaccines were much higher for some events. And the absolute risk of a mild vaccine attributable to AEFIs from the COVID vaccine are very high. In fact, it is likely that children aged 12-15 and adolescents aged 16-25 will experience multiple non-severe adverse events from this COVID vaccine.
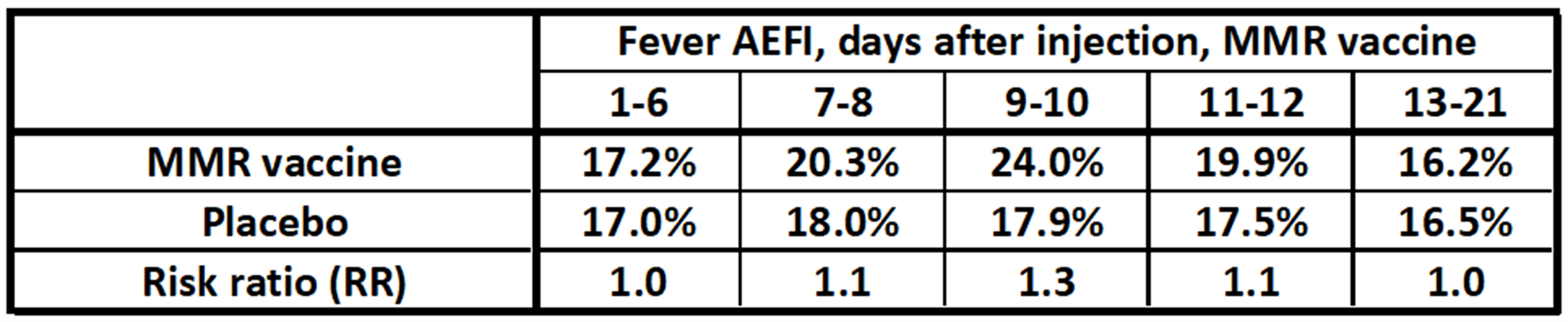
Study of the MMR vaccine and fever symptoms in 581 pairs of children as observed from 0 to 21 days after injection.[30]The MMR vaccine gives a slightly higher percentage of fevers, but the risk ratio of fever was not higher than 1.3 during any interval. Other AEFIs such as respiratory symptoms, nausea, and vomiting were actually higher in the placebo group (meaning the risk ratios were less than 1 for those symptoms).
Conclusions from Study 1: Pfizer’s Phase 3 adolescent trial
No severe adverse events were reported. This is good news but unsurprising given the small size of the study. It is typical of Phase 3 trials of drugs that go on to receive authorization for use.[31]
The volume of mild adverse events was high.
- Mild local events, such as pain at the injection sites were 86% and 83%, respectively for the 12-15 and the 16-25 vaccinated age cohorts.
- Mild systemic events, including fatigue, headache, chills, and muscle pain were common as well, with fatigue and headache occurring in more than 60% of the recipients of Dose 2.
- Fatigue and headaches appear very common in the adolescents tested, and the vaccine contributes to a significant number of additional events.
No material difference in the frequency of AEFIs was reported between age groups.
The mRNA COVID vaccine has a much higher risk ratio for mild AEFIs than in a similar control study with the MMR vaccine. Parents should expect their children of these age groups to experience, on average, more than one mild adverse event from this vaccine. This level of mild AEFIs attributable to the vaccine is deemed as safe enough for approval by the FDA and other approving authorities.[32]
Part II of this analysis of COVID mRNA vaccine AEFIs will examine Studies 2 and 3. It involves millions of individuals and will allow an analysis of more rare and serious adverse events.
References
[1] Frenck, Robert, et al, 2021, Safety, Immunogenicity, and Efficacy of the BNT162b2 Covid-19 Vaccine in Adolescents, N Engl. J. Med, V385, 239-250
[2] Klein, Nicola, et al, 2021, Surveillance for Adverse Events After COVID-19 mRNA Vaccination, JAMA 2021: 326(14) 1390-1399
[3] Barda, Noam, et al, 2021, Safety of the BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting, New England Journal of Medicine, 385: 1078-1090
[4] Witberg, Guy et al., 2021, Myocarditis after Covid-19 Vaccination in a Large Health Care Organization, N. Engl. J. Med.V385, 2132-2139
[5]Mevorach, Dror, et al., 2021, Myocarditis after BNT162b2 mRNA Vaccine against Covid-19 in Israel, N. Engl. J. Med.V385, 2140-2149
[6] Klein, Nicola, et al, 2021, Surveillance for Adverse Events After COVID-19 mRNA Vaccination, JAMA 2021: 326(14) 1390-1399
[7] Halsey, Neal, 2002, The Science of Evaluation of Adverse Events Associated with Vaccination, Seminars in Pediatric Infectious Diseases, V 13, N 3, 205-214
[8] Halsey, Neal, 2002, The Science of Evaluation of Adverse Events Associated with Vaccination, Seminars in Pediatric Infectious Diseases
[9] Halsey, Neal, et al, 2012, Algorithm to assess the causality after individual adverse events following immunizations, Vaccine, V 30 5791-5798
[10] Government of Canada, Adverse events following immunization: Canadian Immunization Guide
[11] Halsey, Neal, 2002, The Science of Evaluation of Adverse Events Associated with Vaccination, Seminars in Pediatric Infectious Diseases, V 13, N 3, 205-214
[12] Halsey, Neal, et al, 2012, Algorithm to assess the causality after individual adverse events following immunizations, Vaccine, V 30 5791-5798
[13] Halsey, Neal, 2002, The Science of Evaluation of Adverse Events Associated with Vaccination, Seminars in Pediatric Infectious Diseases, V 13, N 3, 205-214
[14] Halsey, Neal, et al, 2012, Algorithm to assess the causality after individual adverse events following immunizations, Vaccine, V 30 5791-5798
[15] Halsey, Neal, 2002, The Science of Evaluation of Adverse Events Associated with Vaccination, Seminars in Pediatric Infectious Diseases, V 13, N 3, 205-214
[16] WHO, Poliomyelitis, July 22, 2019
[17] BC Centre for Disease Control, 2009, Relative Risks of Diseases and Immunization
[18] Ward, B. J., Vaccine adverse events in the new millennium: is there a reason for concern? Bulletin of the World Health Organization, V8 N 2, 205-215
[19] Halsey, Neal, 2002, The Science of Evaluation of Adverse Events Associated with Vaccination, Seminars in Pediatric Infectious Diseases, V 13, N 3, 205-214
[20] WHO, Poliomyelitis, July 22, 2019
[21] Halsey, Neal, 2002, The Science of Evaluation of Adverse Events Associated with Vaccination, Seminars in Pediatric Infectious Diseases, V 13, N 3, 205-214
[22] Halsey, Neal, 2002, The Science of Evaluation of Adverse Events Associated with Vaccination, Seminars in Pediatric Infectious Diseases, V 13, N 3, 205-214
[23] WHO, Poliomyelitis, July 22, 2019
[24] BC Centre for Disease Control, 2009, Relative Risks of Diseases and Immunization
[25] Ward, B. J., Vaccine adverse events in the new millennium: is there a reason for concern? Bulletin of the World Health Organization, V8 N 2, 205-215
[26] Frenck, Robert, et al, 2021, Safety, Immunogenicity, and Efficacy of the BNT162b2 Covid-19 Vaccine in Adolescents, N Engl. J. Med, V385, 239-250
[27] Polack, Fernando et al, 2020, Safety and Efficacy of the BNT1622 mRNA Covid-19 Vaccine, New England Journal of Medicine, 2020, 383:2603-2615
[28] Heinonen, Peltola, 1986, Frequency of true adverse reactions to measles-mumps-rubella vaccine. Lancet, V 327, N 8487, 939-942
[29] Heinonen, Peltola, 1986, Frequency of true adverse reactions to measles-mumps-rubella vaccine. Lancet, V 327, N 8487, 939-942
[30] Heinonen, Peltola, 1986, Frequency of true adverse reactions to measles-mumps-rubella vaccine. Lancet, V 327, N 8487, 939-942
[31] Halsey, Neal, 2002, The Science of Evaluation of Adverse Events Associated with Vaccination, Seminars in Pediatric Infectious Diseases, V 13, N 3, 205-214
[32] Frenck, Robert, et al, 2021, Safety, Immunogenicity, and Efficacy of the BNT162b2 Covid-19 Vaccine in Adolescents, N Engl. J. Med, V385, 239-250
(Lee Hunt – BIG Media Ltd., 2021)


